Diseases of finfish
Viral diseases—Viral haemorrhagic septicaemia
CLICK ON IMAGE TO ENLARGE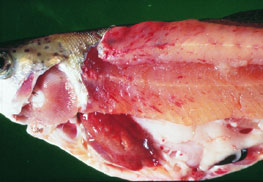
Source: T Håstein
CLICK ON IMAGE TO ENLARGE
Source: T Håstein
Signs of disease
Important: animals with disease may show one or more of the signs below, but disease may still be present in the absence of any signs.
Disease signs at the farm level
Outbreaks are seen in farmed trout and other salmonids, as well as in farmed turbot and Japanese flounder.
Initial outbreak (acute) stage
- high mortality occurs very rapidly
Disease signs at the tank and pond level
Initial outbreak (acute) stage
- lethargy
- separation from shoal
- swimming at pond edges
- Lingering (chronic) stage
- swimming with rotating movement around body axis (ie spinning)
Behavioural (nervous) stage
- low mortality
Clinical signs of disease in an infected animal
Initial outbreak (acute) stage
- slight darkening of body colour
- exophthalmus (pop eye)
- bleeding around eyes
- bleeding under skin around base of pectoral and pelvic fins
- skin ulceration
- pale gills with pinpoint haemorrhages
Lingering (chronic) stage
- intense darkening of skin
- exophthalmus (pop eye)
- gills grey-white (anaemic)
Behavioural (nervous) stage
- most external signs of acute and chronic stages are gone
Gross signs of disease in an infected animal
Initial outbreak (acute) stage
- swollen abdomen, marked by ascites (swollen abdomen from accumulated fluid)
- pinpoint haemorrhages in the fatty tissue, intestine, liver, swim bladder and muscle
Lingering (chronic) stage
- interior of abdomen particularly pale
- liver pale, with evidence of haemorrhages on surface
Behavioural (nervous) stage
- as for acute and chronic stages, above
Disease agent
Viral haemorrhagic septicaemia (VHS) virus is a rhabdovirus of the genus Novirhabdovirus. Several genogroups/genotypes of the virus have been identified from different environments in different parts of the world:
- continental Europe — freshwater group (highly pathogenic to rainbow trout)
- North America — marine group affecting a range of free-living marine and cultured species (highly pathogenic in Pacific herring)
- northern Europe marine strain — marine strain affecting free-living and cultured marine and freshwater species (low pathogenicity in rainbow trout)
- a fourth genotype has been suggested — Snow et al (1999) found isolates from the Baltic Sea that differed from the freshwater or North Sea isolates.
Host range
Fish known to be susceptible to VHS:
Atlantic cod* (Gadus morhua)
Atlantic salmon* (Salmo salar)
blue whiting* (Micromesistius poutassou)
brook trout* (Salvelinus fontinalis)
brown trout* (Salmo trutta)
chinook salmon* (Oncorhynchus tshawytscha)
coho salmon* (Oncorhynchus kisutch)
dab* (Limanda limanda)
English sole* (Pleuronectes vetulus)
European eel* (Anguilla anguilla)
European flounder* (Platichthys flesus)
four-bearded rockling* (Rhinonemus cimbrius)
grayling* (Thymallus thymallus)
Greenland halibut* (Reinhardtius hippoglossoides)
haddock* (Melanogrammus aeglefinus)
herring* (Clupeidae)
Japanese flounder, 'Hirame' strain* (Paralichthys olivaceus)
lesser argentine* (Argentina sphyraena)
North Pacific hake* (Merluccius productus)
Norway pout* (Trisopterus esmarki)
Pacific cod* (Gadus macrocephalus)
Pacific herring* (Clupea pallasi)
Pacific salmon* (Oncorhynchus spp)
Pacific sandlace* (Polygonella myriophylla)
pike* (Esox lucius)
pilchard* (Sardinops sagax)
plaice* (Pleuronectes platessa)
poor cod* (Trisopterus minutus)
rainbow trout* (Oncorhynchus mykiss)
rockling* (Gaidropsarus spp)
sablefish* (Anoplopoma fimbria)
shiner perch* (Cymatogaster aggregata)
Spanish barbel* (Barbus graellsi)
sprat* (Sprattus sprattus)
turbot* (Psetta maxima)
walleye pollock* (Theragra chalcogramma)
whitefish* (Coregonus spp)
whiting* (Merlangius merlangus)
whiting* (Sillago ciliata)
European seabass (Dicentrarchus labrax)
golden trout (Salmo aguabonita)
lake trout (Salvelinus namaycush)
VHS virus can be isolated from marine fish in Europe and the North Pacific (including cod, sprats, herring, haddock and turbot).
* naturally susceptible (other species have been shown to be experimentally susceptible)
Presence in Asia–Pacific
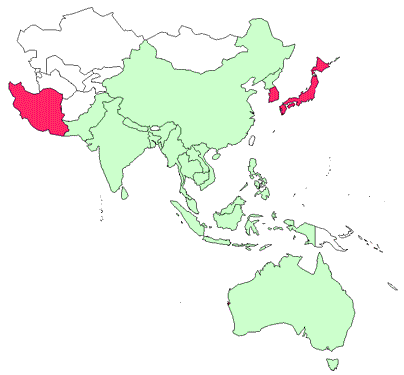
VHS has been officially reported from Iran, Japan and the Republic of Korea.
Epidemiology
- Variant strains of the virus are responsible for disease in different parts of the globe, affecting hosts from different environments.
- Rainbow trout appear to be less susceptible to infection from marine strains of the virus.
- Younger fish are generally more susceptible to disease.
- Water temperature at outbreak is generally between 4°C and 14°C. From 1°C to 5°C, there is an extended course of disease with low daily but high accumulated mortality, whereas temperatures between 14°C to 18°C result in a short disease event with modest mortality.
- Transmission is horizontal directly through the water, from virus shed with faeces, urine (predominantly) and sexual fluids of infected or carrier fish. The virus can also be spread in the faeces of birds that have consumed infected fish and on equipment that has been in contact with water from infected fish.
- Heightened stress caused by overcrowding, extreme temperatures or overfeeding will greatly reduce an animal's resistance to infection.
- Mortality can range from 10% to 80% depending on the stage of disease, water temperature, age of fish and other stressors (highest mortality rates occur in the initial acute stage, with lowest mortality rates in the nervous stage).
- VHS is now thought to have existed in the marine environment before its apparent transfer to freshwater, where it became virulent in trout.
- It has been suggested that the European freshwater isolates of VHS virus originated from fish in the northern Pacific and Atlantic oceans. The mechanism of transfer was possibly through the feeding of marine feed fish to cultured freshwater species (Hedrick et al 2003).
Differential diagnosis
The differential diagnostic table and the list of similar diseases appearing at the bottom of each disease page refer only to the diseases covered by this field guide. Gross signs observed might well be representative of a wider range of diseases not included here. Therefore, these diagnostic aids should not be read as a guide to a definitive diagnosis, but rather as a tool to help identify the listed diseases that most closely account for the gross signs.
Similar diseases
Infectious haematopoietic necrosis, infectious pancreatic necrosis, infectious salmon anaemia
Sample collection
Because of uncertainty in differentiating diseases using only gross signs, and because some aquatic animal disease agents might pose a risk to humans, you should not try to collect samples unless you have been trained. Instead, you should phone your national hotline number and report your observations. If samples have to be collected, the agency taking the call will advise you on what you need to do. Local or district fisheries/veterinary authorities could advise you on sampling.
Emergency disease hotline
For your national emergency disease hotline number, see Whom to contact if you suspect a disease.
Further reading
http://www.oie.int/aac/eng/cards/en_diseasecard.htm
The currently accepted procedures for a conclusive diagnosis of VHS are summarised at http://www.oie.int/eng/normes/fmanual/A_00022.htm
These hyperlinks were correct and functioning at the time of publication.

