Potential for collecting tropical marine fish fry by
light-attraction
John H. Carleton and Peter
J. Doherty
Abstract
Wild fry of marine fishes support a limited range of artisanal grow-out
operations in Asia but this does not apply yet to any high value species of
coral reef fishes. Pelagic juveniles of many reef species can be attracted with
light and AIMS has sampled the supply of these juveniles on the Great Barrier
Reef with anchored and drifting light-traps. The results from two projects (a
two-year study of abundance at cross-shelf scales and a four-year study of
larval supply near two coral reefs) are described with respect to catch
composition and catch rates at family level. The cross-shelf study illustrates
how different taxa use the available habitat space, while the longer study
reveals the temporal (interannual) variations in this fauna. Collectively, the
two studies captured almost 700,000 fishes but the food fishes of highest value
to the Australian market were rare, probably reflecting the representation of
macro-carnivores in natural assemblages. Among these targets, only lethrinids
(emperors) were common enough to warrant further interest. On the basis of
abundance and catchability, the technique has potential to replace more
labour-intensive and destructive methods of collecting some of the more common
ornamental fishes from coral reefs, though its real value may be through
research into the early life histories of wild stocks leading to improved
rearing in artificial environments.
Introduction
With recent evidence that global fish catches are
approaching the theoretical limit of sustainable production from the oceans
(Pauly and Christensen, 1995), aquaculture and sea ranching offer alternative
ways of raising total food production and repairing depleted wild stocks. Both
require reliable supplies of seed and, to date, this has been a major bottleneck
in mariculture. In contrast to some freshwater and estuarine species (e. g.,
Lates calcarifer), the larvae of truly marine fishes, including all high
value tropical reef fishes (e. g., serranids, labrids, lutjanids), have
proven difficult to culture in the numbers required (Lim, 1993; Sorgeloos, 1994).
One way of bypassing the husbandry problems is to
capture wild fry after they have passed through the most difficult stages and
then grow out juveniles. The best example of such an enterprise is the “bangus”
industry of several Asian countries, most notably the Philippines, where
artisanal fishers harvest annually several hundred million juvenile milkfish, Chanos
chanos, from shallow coastal waters with a variety of push nets and weirs
(Kumagai et al., 1980). The same approach is used for culture of
groupers that are collected with attractors made of coconut fronds (Mamauag et
al., 1995). A third method of harvest that offers some potential is
light-attraction (Pascoe, 1990).
Pelagic juveniles of many marine fish larvae are
photopositive during the night, when surface residence may assist their
dispersal (Wolanski et al., 1997). These juveniles are often taken as
by-catch in tuna bait fisheries using lift nets under submerged lights
(Rawlinson, 1990). We have capitalised
on this behaviour to develop automated light-traps as a research tool to
investigate the issues of distribution, abundance and larval supply of a wide
range of tropical fishes on the Great Barrier Reef (GBR). Because these passive traps depend on the
behaviour of the targets, this method is selective for active swimmers (pelagic
juveniles with the full complement of median fins) and is therefore a useful
guide to larval supply (Milicich et al., 1992). The maturity of these animals and their live
state when captured in traps facilitate identification, allowing definition of
the spatial and temporal distribution and abundance of individual species.
In the context of this
workshop, we have two purposes for reviewing data on larvae collected by
light-traps in two sampling projects (one emphasising spatial pattern across
the continental shelf and one emphasising temporal variations in larval supply
at fixed locations). The first is to
establish the constancy and hence reliability of larval supplies on the
GBR. The second is to establish whether
harvesting with light would be a viable method of concentrating the wild fry of
any species suitable for mariculture.
This paper summarises the results of these analyses.
Materials and Methods
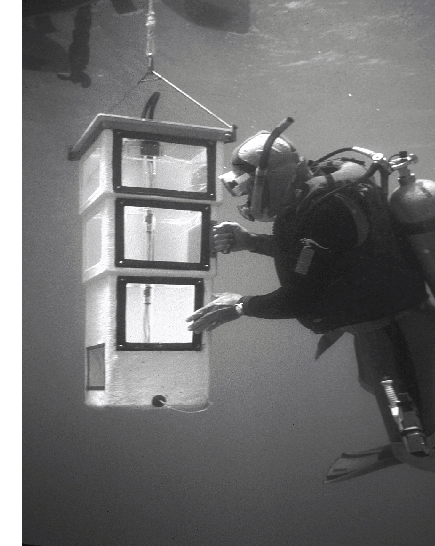
All sampling was based
on submerged light-traps (Figure 1) similar to those described by Doherty
(1987). These traps have no moving parts and depend upon attracting
photopositive organisms with slow flashing lights through two baffled chambers
into a third where most of the catch remains alive until collected.
Operation of the lights is automated, which can allow
arrays of traps to reveal synoptic views of spatial pattern. With daily
clearance, anchored traps also can yield extensive time series of data
(Milicich 1992).
Our
general protocol for fixed (anchored) traps was to show light over three
periods during each night (2100-2200, 2400-0100, 0300-0400), partly to conserve
battery- life for extended sampling, but also partly to spread tidal and diel
effects more evenly among nights of sampling (Doherty 1987). In addition, most sampling was concentrated
around new moons between October and February when seasonal and lunar spawning
patterns produce the largest catches of coral reef fishes (Doherty 1991).
Cross Shelf Study
In two consecutive
years, we anchored light-traps immediately downstream (within 100 m) of
four coral reefs located between 50 and 110 km off the coast of Townsville
(Figure 2) and sampled them daily for at least a week around each of five
consecutive new moon periods. In 1990/91, each reef was sampled by three traps
suspended near the surface and one trap suspended at 20 m depth from the
middle mooring. In 1991/92, two deep traps were suspended from the outside moorings
in addition to the three surface traps (Table 1).
During the same nights,
we also sampled open water habitats with free light-traps that were allowed to
drift for one-hour periods at 15 stations between the coast and the Coral Sea
(Figure 2). Each such release involved an independent pair of shallow and deep
traps. In 1990/91, all deep drifting traps were limited to a maximum of 20 m
to match those on the reef. In 1991/92, the deep traps were suspended at
5 m above the bottom or to a maximum depth of 100 m.
All sampling was done
between 2000-0500H, which with transit time allowed five consecutive stations
to be sampled per night. Consequently, one night transect consisted of samples
from the GBR Lagoon (stations 1-5), Magnetic Passage (stations 6-10) or Coral
Sea (stations 11-15). Whenever possible, we sampled each shelf station three
times during a cruise. Lower replication was attempted in the Coral Sea
(Table 1) due to the persistent low catches of coastal fish larvae beyond
the continental margin.
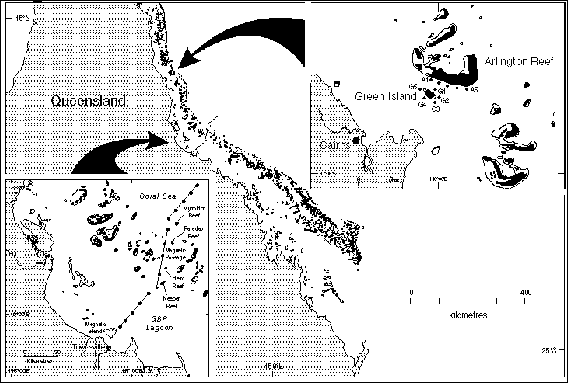
Figure 2. Map
of study sites
Table 1. Sampling program and fishing effort (in
light-trap hours) by major habitat in the cross-shelf study
|
Habitat
|
|
Trap
|
1990/91
|
1991/92
|
|
Stations
|
Deployment
|
Depth (m)
|
Replicates
|
Effort
|
Depth (m)
|
Replicates
|
Effort
|
|
GBR Lagoon
|
5
|
Drifting
|
0
|
2
|
102
|
0
|
2
|
119
|
|
|
|
|
20
|
2
|
102
|
Near bottom
|
2
|
120
|
|
Magnetic
Passage
|
5
|
Drifting
|
0
|
2
|
81
|
0
|
2
|
90
|
|
|
|
|
20
|
2
|
81
|
Near bottom
|
2
|
88
|
|
Coral
Sea
|
5
|
Drifting
|
0
|
2
|
71
|
0
|
2
|
40
|
|
|
|
|
20
|
2
|
72
|
100
|
2
|
39
|
|
Reefs
|
3
|
Anchored
|
0
|
*3
|
724
|
0
|
3
|
996
|
|
|
|
|
20
|
1
|
228
|
20
|
2
|
627
|
*Only two surface traps were deployed during the first cruise in
October 1990.
Larval supply study
Starting in 1990/91, we
monitored larval supply over four years around two reefs off Cairns (Arlington,
Green) with depth-stratified anchored arrays of traps (Figure 2). All traps were cleared daily when possible
(exceptions were discarded) and sampled for at least 10 days around five
consecutive new moons matching those in the cross-shelf study.
Although the distribution of effort varied among years
(Table 2), we tried to balance effort between depths within reefs and years,
with the exception of 1993/94. However, information from three prior seasons of
sampling elsewhere confirmed that the pelagic juveniles of benthic reef species
are most common near the surface. We
then abandoned deep-sampling in the final year in order to achieve a
more-balanced effort between reefs and to provide replication at locations
within reefs.
Table 2.
Sampling program and fishing effort (in light-trap hours) at Arlington
and Green Reefs during the larval supply study
|
|
1990/91
|
1991/92
|
1992/93
|
1993/94
|
|
|
Sites
|
Depth
|
Repl
|
Effort
|
Sites
|
Depth
|
Repl
|
Effort
|
Sites
|
Depth
|
Repl
|
Effort
|
Sites
|
Depth
|
Repl
|
Effort
|
|
Arlington
|
4
|
1
|
1
|
618
|
5
|
1
|
1
|
888
|
5
|
1
|
1
|
888
|
5
|
1
|
2
|
1221
|
|
|
|
20
|
1
|
558
|
|
20
|
1
|
663
|
|
20
|
1
|
774
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Green
|
3
|
1
|
1
|
516
|
3
|
1
|
1
|
399
|
3
|
1
|
1
|
507
|
5
|
1
|
2
|
1212
|
|
|
|
20
|
1
|
354
|
|
20
|
1
|
258
|
|
20
|
1
|
357
|
|
|
|
|
Results
Cross Shelf Study
Over two spawning
seasons, we collected samples of pelagic juvenile fish with >3,500 hours of
light representing >1000 samples from open water and >850 samples from
the leeward sides of the four coral reefs (Table 1). All samples represent collection from a site and depth
combination; the difference in total hours of light between reef and non-reef
habitats is explained by the longer operation of anchored traps (Table 1). To
facilitate uniform comparisons, most of the following data have been
standardised to catch-per-hour of light.
In total, we collected
>130,000 fish consisting of >600 recognisable types belonging to >70
families with many individual distributional patterns. The strongest pattern was related to depth
with the majority of families having higher catch rates near the surface (Table
2). A number of exceptions (with higher
catch rates in deep traps) were encountered.
Of those with sufficient numbers to be convincing, most were pelagic
fishes belonging to the families Scombridae, Carangidae, Engraulidae. In each case, this pattern was driven by the
abundance of one dominant genus: Rastrelliger, Decapterus, and
Stolephorus, respectively. Only a
few families of benthic fishes (e. g., Synodontidae, Priacanthidae, Trichiuridae)
were more abundant near the bottom.
Drifting traps in open water habitats collected all of these
deep-dwelling taxa.
After standardisation of
effort, pelagic juveniles of coral reef fishes were captured in greatest
numbers by the traps anchored near coral reefs. Approximately half of these fishes were a mixture of larvae,
juveniles, and adults of two species of small sprat (Spratelloides delicatulus
and S. gracilis), that are known to complete their lifecycles in
coral reef lagoons (Leis 1994).
Although the overall abundance of these fishes remained constant across
the two years (Clupeidae, Table 2), their proportional representation in
shallow samples decreased from 64% in 1990/91 to 40% in 1991/92. This reduction
in dominance was caused by significantly greater catches of reef species during
the second year. This increase was
coherent across many of the non-clupeid taxa and is illustrated here with the
catch rates for the second and third most abundant taxa: the damselfish
subfamilies Pomacentrinae and Chrominae (Figure 3). Whereas the former is a diverse assemblage of almost 100 species,
making interpretation ambiguous, the Chrominae were dominated by a single
species, Chromis atripectoralis. Thus, it appears that many species were
affected in similar manner as a result of some widespread influence. Furthermore, the temporal changes were
consistent for both groups on three of the four coral reefs (exception Helix
Reef) spanning the full width of the reef matrix. After adjustment of the different catch rates among taxa and
years, the distribution of abundance among the three reefs (excluding Helix)
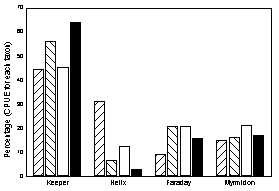
was remarkably consistent with greatest abundance at Keeper Reef (Figure 4).
Consistent spatial
patterns were detected among most of the major taxa collected with drifting
traps from open water habitats, including the low catches of shelf species from
all stations beyond the continental margin mentioned earlier. We illustrate the
range of patterns observed on the shelf with four examples (Figure 5).
Lethrinids were most common in surface samples from the GBR Lagoon, whereas Rastrelliger
was most abundant near the bottom in the same major habitat. Decapterus was also most common near
the bottom but was found in greatest abundance at stations further offshore in
the Magnetic Passage. Mackerels, belonging to the genus Scomberomorus,
were most common in the GBR Lagoon but without such clear preference for depth.
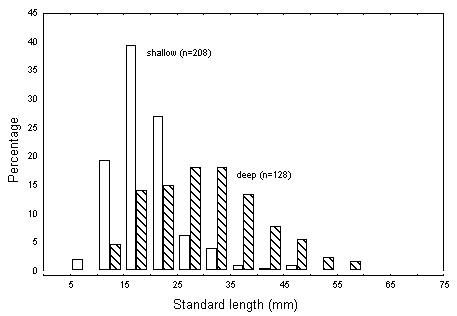
Further dissection of their catches, however, showed a difference in the size
of individuals captured near the surface and bottom (Figure 6) indicating a
possible ontogenetic change in the use of space.
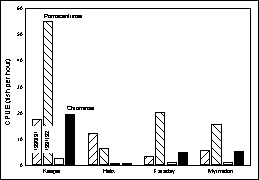
Figure 3. Catch rates of two subfamilies of reef fish at
four coral reefs.
Figure 4. Relative distribution of the two subfamilies
among the four reefs. Shading patterns as in the previous Figure 3.
Larval
Supply Study
Over four spawning
seasons, we collected >500,000 pelagic juvenile fish (Table 4) with
>9,000 hours of light (>3,000 samples) from multiple traps anchored close
to the two reefs. Because of the lack
of “deep” effort in the final year and the much lower catches near the bottom,
we focus here only on fishes caught near the surface.
As in the cross-shelf
study, temporal trends were strong. Catches at individual traps varied among
days, months, and years. We have pooled
the first two scales (as representing only patchiness and spawning cycles) and
report the interannual variation as the factor most likely to affect population
dynamics and any attempts to harvest natural supplies of fish fry.
In contrast to the
observations on the four reefs off Townsville, there was no increase between
1990/91 and 1991/92 in the CPUE of the Pomacentrinae or Chrominae on either
reef (Tables 5, 6). In the third
season, collections from both reefs were dominated (~70%) by an influx of
Pomacentrinae but Chrominae did not change in a similar way suggesting that the
patterns were driven at the level of species.
This pattern of independent changes was repeated among the dominant
non-clupeid taxa within and between reefs (Tables 5, 6). The blue sprat (Spratelloides delicatulus)
was again captured in great numbers (49% of total catch on Arlington - rank 1;
32% of total from Green - rank 2).
Catch rates of this species varied by more than an order of magnitude
among years but without coherence among the two adjacent populations.
We attribute these differences to local influences
given their closed life cycles.
In contrast to the
incoherent temporal patterns among taxa within and between reefs, one spatial
effect was obvious besides the previously reported effect of depth. Catch rates
for the 10 most abundant species were all greater from traps set near Arlington
than Green, though this may reflect their different positioning around the two
reefs. While such positional effects
can determine the absolute catch rates of individual taxa, the most desirable
targets (species with high unit value attractive to commercial fishers) were
always rare, which simply may reflect their natural representation in reef
communities
Discussion
A comprehensive understanding of the ecology of
presettlement tropical fishes has been elusive in the past; partly because of
the high costs of sampling this notoriously patchy fauna and also partly because
of a failure of suitable methods.
Traditional methods of sampling plankton, which rely upon towed nets,
may capture large numbers of individuals but their catches are biased towards
minute preflexion (no tail formation) stages that are difficult to identify and
clearly are not viable to culture. Until the advent of alternative sampling
methods, the scarcity of the more mature and robust stages could be attributed
to extremely high rates of natural mortality in the ocean, but the deployment
of light-traps (Doherty 1987) in the same water has shown that the larger
stages are agile swimmers that simply avoid conventional gears (Choat et al.,
1993).
Light-traps were
developed as tools to study spatial and temporal variations in the supply of
pelagic juveniles to coral reef habitats and their catches have been shown to
correlate well with the replenishment of benthic populations (Milicich,
1992). This should not be surprising
because the efficiency of these passive devices depends upon the active orientation
(photopositive behaviour and swimming ability) of the targets. Lack of either capacity means that
light-traps will be selective, obviously undersampling fish that are not
attracted to light (taxonomic selectivity) and feeble swimmers regardless of their
attraction (size-selective bias).
The inherent
size-selectivity of light-traps is an advantage for certain studies. Since most
of the fish caught are large, agile swimmers with fully-developed fins, much of
the catch can be identified to species level, which allows better ecological
study. Since we still have a
rudimentary knowledge of the early life histories of most tropical fishes,
better field studies could assist with attempts to close their lifecycles in hatcheries
by defining the dietary and water quality requirements of natural populations.
In the short-term, there is potential also to consider light-trapping as an
alternative method for collecting wild fry since the majority of fish captured
by this technique are alive when collected and robust enough to be candidates
for mariculture.
Table
3. Depth-stratified catch rates
(and relative abundance) from the cross-shelf study. Percentages of <0.01
after rounding were suppressed to increase readability of the table.
|
Families
|
1990/91
|
1991/92
|
Total
|
|
|
Shallow
|
Deep
|
Shallow
|
Deep
|
Shallow
|
Deep
|
|
Clupeidae
|
27.59
|
(63.49)
|
2.67
|
(32.57)
|
24.26
|
(39.84)
|
4.48
|
(31.00)
|
25.72
|
(48.29)
|
3.83
|
(31.38)
|
|
Pomacentrinae
|
8.71
|
(20.05)
|
0.56
|
(6.79)
|
21.74
|
(35.70)
|
0.44
|
(3.06)
|
16.03
|
(30.11)
|
0.48
|
(3.95)
|
|
Chrominae
|
1.2
|
(2.77)
|
0.04
|
(0.43)
|
6.42
|
(10.54)
|
0.2
|
(1.40)
|
4.13
|
(7.76)
|
0.14
|
(1.17)
|
|
Apogonidae
|
1.82
|
(4.20)
|
0.45
|
(5.42)
|
2.46
|
(4.03)
|
0.98
|
(6.81)
|
2.18
|
(4.09)
|
0.79
|
(6.48)
|
|
Blenniidae
|
0.48
|
(1.10)
|
0.07
|
(0.88)
|
1.48
|
(2.44)
|
0.17
|
(1.18)
|
1.04
|
(1.96)
|
0.14
|
(1.11)
|
|
Monocanthinae
|
0.56
|
(1.28)
|
0.16
|
(1.92)
|
0.83
|
(1.37)
|
0.12
|
(0.83)
|
0.71
|
(1.34)
|
0.13
|
(1.09)
|
|
Atherinidae
|
0.56
|
(1.29)
|
|
|
0.64
|
(1.05)
|
0.003
|
(0.02)
|
0.6
|
(1.13)
|
0.002
|
(0.02)
|
|
*
Scombridae
|
0.36
|
(0.82)
|
1.37
|
(16.75)
|
0.56
|
(0.92)
|
1.85
|
(12.83)
|
0.47
|
(0.89)
|
1.68
|
(13.77)
|
|
Gobiidae
|
0.14
|
(0.32)
|
0.15
|
(1.87)
|
0.66
|
(1.08)
|
0.29
|
(1.98)
|
0.43
|
(0.80)
|
0.24
|
(1.95)
|
|
*
Carangidae
|
0.25
|
(0.58)
|
0.74
|
(9.01)
|
0.25
|
(0.41)
|
1.36
|
(9.42)
|
0.25
|
(0.47)
|
1.14
|
(9.32)
|
|
Holocentridae
|
0.4
|
(0.92)
|
0.08
|
(0.93)
|
0.1
|
(0.16)
|
0.002
|
(0.02)
|
0.23
|
(0.43)
|
0.03
|
(0.23)
|
|
Nomeidae
|
0.24
|
(0.55)
|
0.008
|
(0.10)
|
0.16
|
(0.26)
|
0.008
|
(0.06)
|
0.19
|
(0.36)
|
0.008
|
(0.07)
|
|
Myctophidae
|
0.33
|
(0.76)
|
0.02
|
(0.30)
|
0.04
|
(0.06)
|
0.02
|
(0.12)
|
0.17
|
(0.31)
|
0.02
|
(0.16)
|
|
Engraulidae
|
0.06
|
(0.15)
|
0.41
|
(4.97)
|
0.2
|
(0.32)
|
2.18
|
(15.09)
|
0.14
|
(0.26)
|
1.55
|
(12.67)
|
|
Bregmacerotidae
|
0.05
|
(0.11)
|
0.3
|
(3.660
|
0.18
|
(0.29)
|
0.17
|
(1.20)
|
0.12
|
(0.23)
|
0.22
|
(1.78)
|
|
Labridae
|
0.12
|
(0.28)
|
0.04
|
(0.43)
|
0.13
|
(0.21)
|
0.01
|
(0.09)
|
0.12
|
(0.23)
|
0.02
|
(0.17)
|
|
*
Lethrinidae
|
0.12
|
(0.29)
|
0.008
|
(0.10)
|
0.12
|
(0.19)
|
0.02
|
(0.13)
|
0.12
|
(0.23)
|
0.01
|
(0.12)
|
|
*
Siganidae
|
0.06
|
(0.13)
|
0.06
|
(0.68)
|
0.15
|
(0.24)
|
0.12
|
(0.86)
|
0.11
|
(0.20)
|
0.1
|
(0.82)
|
|
Scorpaeninae
|
0.04
|
(0.09)
|
0.004
|
(0.05)
|
0.11
|
(0.19)
|
0.009
|
(0.06)
|
0.08
|
(0.15)
|
0.007
|
(0.06)
|
|
Synodontidae
|
0.04
|
(0.09)
|
0.26
|
(3.20)
|
0.09
|
(0.14)
|
0.46
|
(3.19)
|
0.07
|
(0.12)
|
0.39
|
(3.20)
|
|
*
Mullidae
|
0.05
|
(0.11)
|
0.09
|
(1.06)
|
0.05
|
(0.08)
|
0.02
|
(0.15)
|
0.05
|
(0.09)
|
0.04
|
(0.37)
|
|
Syngnathidae
|
0.06
|
(0.15)
|
0.004
|
(0.05)
|
0.02
|
(0.03)
|
0.01
|
(0.07)
|
0.04
|
(0.07)
|
0.008
|
(0.07)
|
|
Chaetodontidae
|
0.01
|
(0.03)
|
0.004
|
(0.05)
|
0.04
|
(0.06)
|
0.006
|
(0.04)
|
0.03
|
(0.05)
|
0.005
|
(0.04)
|
|
Dactylopteridae
|
0.02
|
(0.04)
|
0.008
|
(0.10)
|
0.01
|
(0.02)
|
0.002
|
(0.02)
|
0.02
|
(0.03)
|
0.004
|
(0.04)
|
|
*
Nemipteridae
|
0.003
|
(0.01)
|
|
|
0.03
|
(0.05)
|
0.002
|
(0.02)
|
0.02
|
(0.04)
|
0.001
|
(0.01)
|
|
Pseudochromidae
|
0.01
|
(0.03)
|
0.02
|
(0.28)
|
0.03
|
(0.05)
|
0.37
|
(2.57)
|
0.02
|
(0.04)
|
0.25
|
(2.02)
|
|
Priacanthidae
|
0.03
|
(0.06)
|
0.23
|
(2.83)
|
0.01
|
(0.02)
|
0.41
|
(2.84)
|
0.02
|
(0.04)
|
0.35
|
(2.84)
|
|
*
Caesionidae
|
0.04
|
(0.10)
|
0.04
|
(0.48)
|
0.006
|
(0.01)
|
0.09
|
(0.63)
|
0.02
|
(0.04)
|
0.07
|
(0.60)
|
|
Plesiopidae
|
0.005
|
(0.01)
|
0.03
|
(0.40)
|
0.01
|
(0.02)
|
0.003
|
(0.02)
|
0.01
|
(0.02)
|
0.01
|
(0.11)
|
|
Sphyraenidae
|
0.02
|
(0.05)
|
|
|
0.003
|
(0.01)
|
0.003
|
(0.02)
|
0.01
|
(0.02)
|
0.002
|
(0.02)
|
|
*
Serranidae
|
0.009
|
(0.02)
|
0.006
|
(0.08)
|
0.01
|
(0.02)
|
0.03
|
(0.21)
|
0.01
|
(0.02)
|
0.02
|
(0.18)
|
|
Schindleriidae
|
0.001
|
|
0.02
|
(0.20)
|
0.02
|
(0.04)
|
0.17
|
(1.18)
|
0.01
|
(0.03)
|
0.12
|
(0.95)
|
|
Pseudoplesiopidae
|
0.004
|
(0.01)
|
0.01
|
(0.13)
|
0.01
|
(0.02)
|
0.006
|
(0.04)
|
0.008
|
(0.01)
|
0.007
|
(0.06)
|
|
Canthigasterinae
|
0.003
|
(0.01)
|
|
|
0.009
|
(0.01)
|
|
|
0.006
|
(0.01)
|
|
|
|
Microdesmidae
|
|
|
0.002
|
(0.03)
|
0.01
|
(0.02)
|
0.23
|
(1.60)
|
0.005
|
(0.01)
|
0.15
|
(1.22)
|
|
Amphiprioninae
|
0.003
|
(0.01)
|
|
|
0.006
|
(0.01)
|
|
|
0.005
|
(0.01)
|
|
|
Table 3
cont'd.
|
Families
|
1990/91
|
1991/92
|
Total
|
|
|
Shallow
|
Deep
|
Shallow
|
Deep
|
Shallow
|
Deep
|
|
Eleotridae
|
0.001
|
|
|
|
0.006
|
(0.01)
|
0.002
|
(0.02)
|
0.004
|
(0.01)
|
0.001
|
(0.01)
|
|
Acanthuridae
|
0.001
|
|
0.01
|
(0.13)
|
0.006
|
(0.01)
|
0.04
|
(0.27)
|
0.004
|
(0.01)
|
0.03
|
(0.23)
|
|
Grammistidae
|
0.007
|
(0.02)
|
0.004
|
(0.05)
|
0.002
|
|
0.003
|
(0.02)
|
0.004
|
(0.01)
|
0.004
|
(0.03)
|
|
Gerreidae
|
0.005
|
(0.01)
|
0.002
|
(0.03)
|
0.002
|
|
0.009
|
(0.06)
|
0.004
|
(0.01)
|
0.007
|
(0.05)
|
|
Scaridae
|
|
|
|
|
0.005
|
(0.01)
|
|
|
0.003
|
(0.01)
|
|
|
|
Diodontidae
|
0.004
|
(0.01)
|
|
|
0.002
|
|
|
|
0.003
|
(0.01)
|
|
|
|
Hemiramphidae
|
0.007
|
(0.02)
|
|
|
|
|
|
|
0.003
|
(0.01)
|
|
|
|
Balistinae
|
0.001
|
|
|
|
0.002
|
|
|
|
0.002
|
|
|
|
|
Bothidae
|
0.001
|
|
0.006
|
(0.08)
|
0.002
|
|
0.002
|
(0.02)
|
0.002
|
|
0.004
|
(0.03)
|
|
Istiophoridae
|
0.004
|
(0.01)
|
0.002
|
(0.03)
|
|
|
|
|
0.002
|
|
0.0007
|
(0.01)
|
|
Teraponidae
|
0.002
|
(0.01)
|
|
|
0.002
|
|
|
|
0.002
|
|
|
|
|
Muraenidae
|
0.001
|
|
|
|
0.002
|
|
|
|
0.002
|
|
|
|
|
*
Lutjanidae
|
0.003
|
(0.01)
|
0.03
|
(0.40)
|
0.002
|
|
0.006
|
(0.04)
|
0.002
|
|
0.02
|
(0.13)
|
|
Ostraciidae
|
0.002
|
(0.01)
|
|
|
0.002
|
|
|
|
0.002
|
|
|
|
|
Pempheridae
|
|
|
0.01
|
(0.15)
|
0.003
|
(0.01)
|
0.04
|
(0.26)
|
0.002
|
|
0.03
|
(0.23)
|
|
Anguillidae
|
0.001
|
|
|
|
0.002
|
|
|
|
0.001
|
|
|
|
|
Gobiesocidae
|
0.003
|
(0.01)
|
0.002
|
(0.03)
|
|
|
|
|
0.001
|
|
0.0007
|
(0.01)
|
|
Ophichthidae
|
0.002
|
(0.01)
|
|
|
0.0008
|
|
|
|
0.001
|
|
|
|
|
Exocoetidae
|
0.001
|
|
|
|
0.0008
|
|
|
|
0.001
|
|
|
|
|
Pomacanthidae
|
0.001
|
|
0.02
|
(0.20)
|
0.0008
|
|
0.005
|
(0.03)
|
0.001
|
|
0.009
|
(0.07)
|
|
Bythitidae
|
|
|
|
|
0.002
|
|
|
|
0.001
|
|
|
|
|
Tetraodontinae
|
0.003
|
(0.01)
|
0.006
|
(0.08)
|
|
|
0.001
|
(0.01)
|
0.001
|
|
0.003
|
(0.02)
|
|
Uranoscopidae
|
0.001
|
|
|
|
0.0008
|
|
0.001
|
(0.01)
|
0.001
|
|
0.0007
|
(0.01)
|
|
Cirrhitidae
|
|
|
|
|
0.0008
|
|
|
|
0.0005
|
|
|
|
|
Congrogadidae
|
|
|
|
|
0.0008
|
|
|
|
0.0005
|
|
|
|
|
Trichiuridae
|
|
|
0.24
|
(2.93)
|
0.0008
|
|
0.06
|
(0.42)
|
0.0005
|
|
0.12
|
(1.02)
|
|
Ephippidae
|
|
|
|
|
0.0008
|
|
|
|
0.0005
|
|
|
|
|
Kyphosidae
|
0.001
|
|
|
|
|
|
|
|
0.0005
|
|
|
|
|
Pseudogrammidae
|
|
|
|
|
|
|
0.001
|
(0.01)
|
|
|
0.0007
|
(0.01)
|
|
Plotosidae
|
|
|
|
|
|
|
0.001
|
(0.01)
|
|
|
0.0007
|
(0.01)
|
|
Leiognathidae
|
|
|
0.008
|
(0.10)
|
|
|
0.003
|
(0.02)
|
|
|
0.005
|
(0.04)
|
|
Centriscidae
|
|
|
0.002
|
(0.03)
|
|
|
|
|
|
|
0.0007
|
(0.01)
|
|
Congridae
|
|
|
|
|
|
|
0.001
|
(0.01)
|
|
|
0.0007
|
(0.01)
|
|
Gempylidae
|
|
|
0.002
|
(0.03)
|
|
|
0.003
|
(0.02)
|
|
|
0.003
|
(0.02)
|
|
Mugiloididae
|
|
|
|
|
|
|
0.001
|
(0.01)
|
|
|
0.0007
|
(0.01)
|
|
Total
fish
|
41843
|
|
3963
|
|
75268
|
|
12618
|
|
117111
|
|
16581
|
|
* families of maximum interest as sources of food
We have grown-out various fish caught with traps where
their identification were ambiguous at the time of collection. For example, the larval supply study
described above was done for the purpose of monitoring the replenishment of the
most valuable fish taken by commercial and recreational fishers on the Great
Barrier Reef - coral trouts belonging to the genus, Plectropomus
(Doherty et al. , 1994). During
our study, we collected approximately 650 individuals from this species complex
but all looked alike when collected due to their intense red pigmentation.
During the course of the project, we transferred several hundred live
individuals to culture facilities on the mainland, where, after initial
problems with water quality, some were grown-up to one-year-old juveniles
confirming that the catches were dominated by Plectropomus leopardus. It
was this experience that raised our expectations that wild fry collected by
light attraction might be used to circumvent the technical problems that have
been encountered in closing the life cycles of high value reef fishes in the
laboratory. Although our intention has
never been to substitute for artificial mass culture, it raises the question of
whether the fry of any species attractive to mariculture is so abundant in the
wild that it could be harvested in numbers to support a growing industry.
To answer this question,
we have summarised the results of two sampling exercises that collected almost
700,000 fish. The first project
sampled fish across the continental shelf off Townsville in habitats both near
and far from coral reefs. Our reason
for this study was to identify the use of habitat space by a wide range of reef
and coastal fishes. In the limited
number of examples presented here, we have shown that different species may
have unique requirements, some of which may also vary during the ontogeny of
that species. Such information allows
future sampling to be better targeted to places of maximum abundance. The second project sampled fish from near two
coral reefs, where our intention was to monitor temporal variations in larval
supply in order to examine coupling between the availability of propagules and
the replenishment of local populations.
From the perspective of this conference, which is concerned with the
culture of marine fishes, it provides data on the availability of attractive
targets among years.
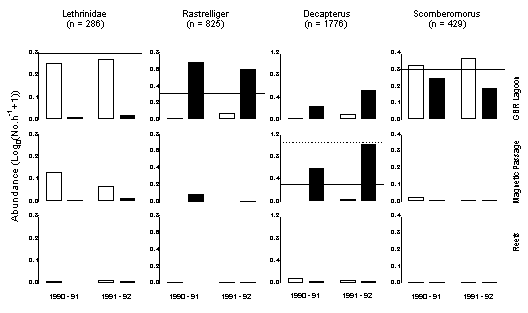
Figure
5. Catch rates from the three major
shelf habitats (GBR Lagoon, Magnetic Passage, coral reefs) of four taxa with
contrasting distributions. Shading identifies catch rates from shallow
(unfilled) and deep (filled) traps respectively. The horizontal lines identify
back-transformed catch rates of one (unbroken) and 10 (broken) fish per hour of
effort.
Figure 6.
Size-frequency distributions of mackerels in the genus, Scomberomorus,
in deep and shallow samples from the Great Barrier Reef Lagoon.
|
While recognising that
the GBR is not necessarily a model for other countries, our results can be
summarised briefly here for the purpose of this conference. Our cross-shelf study was completed at
considerable cost to the Australian Government because it covered deep and
shallow habitats up to 150 km from the coast.
While it revealed a lot of new information about the distribution and
abundance of the early life history stages of a wide range of coastal fishes,
it did not reveal an untapped source of juveniles suitable for cost-effective
culture. The possible exception is that
of the lethrinids (emperors), which were most abundant in surface waters near
the coast. The data that we have shown
may not be particularly convincing because we caught fewer than 300 individuals
from around 100 hours of light-trapping in the GBR Lagoon though returns were
considerably higher from specific locations.
In a third season of sampling in this habitat (data not reported here),
catch rates of lethrinids were approximately 4 times greater. This example is meant to make two
points. First, catch-per-unit effort
can be maximised by focussed effort (a fact long exploited by fishermen with
their traditional knowledge of the habits of fish) but it will vary among
spawning seasons due to a range of environmental influences on larval transport
and survival.
The cross-shelf study,
sampling both near and far from coral reefs, showed that the most effective
place to collect the pelagic juveniles of reef-associated species is at the
surface in the lee of coral reefs (Doherty and Carleton in press). We believe that this concentration factor
arises from the combination of hydrodynamic forces and the active orientation
of presettlement reef fishes (Wolanski et al., 1997). We also believe, on the preliminary data
presented here, that the size of the reef has an effect on this concentration
factor. The evidence affecting this
belief is the poor and erratic larval supply sampled at Helix Reef (the
smallest and most isolated reef in our sampling) in the cross-shelf study
against the greater catch rates from traps placed downstream of the huge and
extensive Arlington Reef. The latter extends
across most of the outer shelf off Cairns.
Such an effect is consistent with the lower catch rates observed at the
neighbouring Green Reef, although without replication, we cannot be certain
that the larger reef was not starving its neighbour of recruits by its upstream
position. In any case, we suggest that
large reefs will be the best places to seek pelagic propagules in useful
concentrations, though we caution that this result may pertain only to reefs
subject to strong hydrodynamic flows like those of the GBR.
Accepting that reefs
with the characteristics of Arlington may be optimal places from which to
harvest the fry of valuable reef fishes, we still conclude that such an
enterprise would be a risky business.
During our four-year study at Arlington, the catches of the high-value
coral trouts ranged from 339 to 27, with the least number of juveniles
collected during the year of greatest effort (Table 2). Interannual variability
in larval supply comes as no surprise to students of fish population dynamics
but, at such levels, it poses an unacceptable risk to any commercial
venture. While this risk could be
spread by not concentrating on a single species, we note that all of the
potential target species that is considered most valuable in Australia (mostly
fish of high trophic status) were rare.
This may reflect nothing other than the natural representation of
macrocarnivores in reef fish communities but, separate from the economic
considerations, conservation imperatives in Australia would rule such
harvesting out of the question because of the extensive by-catch.
Based on abundance
alone, it would make more sense to exploit the resource for ornamental reef
fishes (Andrews 1990) and this may be a valuable niche market. Though
preliminary trials with pomacentrids suggest that their larviculture is not
particularly challenging, due to the large size of the hatchlings emerging from
demersal eggs. The experience of a few
companies that have bred tropical marine fishes for the aquarium trade is that
their product is uncompetitive and commands low prices due to the drab colours
of cultured juveniles compared with those from wild stocks (Anon., 1992). These
factors explain why the aquarium trade in marine fishes remains almost totally
reliant on the collection of individuals from the wild despite the technical
capacity to culture some species. While
the unit values of common species are relatively low, the catch rates that are
possible through light-attraction (>30,000
damselfishes in one night, Milicich, 1992) suggest that such harvesting may be
more effective and less destructive than present techniques for collecting the
same species from reef habitats.
In the case of food
fishes, after a vast amount of effort and a substantial number of dead fish, we
can look only to two families (Siganidae, Lethrinidae) with any interest for
this audience. The rabbitfishes are of
little interest at present to the Australian market but the recent successes
with their mass- culture in Asia challenges the need for expanded harvest of
their wild fry. Until a real shortage
of reef fishes places a higher unit value on alternative species, we can point
only to the catch rates of emperors (family: Lethrinidae) as a possible
cost-effective target for the harvest of wild fry. These fish (attractive to the Australian market) have been
harvested in large numbers in several of our sampling projects (often as much
as 25% of the catch - see also Milicich and Doherty, 1994), have proven to be
resilient in captivity and have shown excellent growth rates.
Acknowledgement
We thank the crews of the RV Lady Basten for
logistic support; John Collingwood for trap manufacture; Helen Sturmey, Kim
Smith, Chad Lunow for sorting thousands of samples; David Harris, Phillip
Light, and volunteers for maintaining the extended sampling in the Cairns
study; Tara Anderson for the preliminary analysis of data from the cross-shelf
study. This is AIMS Contribution no.
879.
Literature Cited
Andrews,
C. 1990. The ornamental fish trade and fish conservation. J. Fish. Biol. 37: 53-59.
Anon., 1992. Marine ornamental fish: world trade. Aqua. Farm News X (1): 1-5.
Carleton, J.
H. and P. J. Doherty. 1997. The distribution and abundance of pelagic
juvenile fish near Grub Reef, central Great Barrier Reef. In: Lession, H. A.
and I. G. MacIntyre. Proc. 8th Int.
Coral Reef Symp. 1:1155-1160.
Choat, J. H., P. J. Doherty, B. A. Kerrigan, and J. M. Leis. 1993.
A comparison of towed nets, purse seine and light aggregation devices
for sampling larvae and pelagic juveniles of coral reef fishes. Fish. Bull.
91:195-209.
Doherty, P.
J. 1987. Light-traps: selective but useful devices for quantifying the
distributions and abundances of larval fishes.
Bull. Mar. Sci.
41:423-431.
Doherty, P.
J. 1991. Spatial and temporal patterns in recruitment. pp. 261-293 In: Sale, P. F. (ed.).
The ecology of coral reef fishes.
NY: Academic Press.
Doherty, P.
J., A. J. Fowler, M. A. Samoilys, and D. A. Harris. 1994. Monitoring the
replenishment of coral trout (Pisces: Serranidae) populations. Bull.
Mar. Sci. 54: 343-355.
Kumagai, S., T. Bagarinao, and A. Unggui. 1980.
A study on the milkfish fry fishing gears in Panay Island, Philippines. SEAFDEC Tech. Rep. 6.
Leis, J.
M. 1994. Coral Sea atoll lagoons: Closed nurseries for the larvae of a few
coral reef fishes. Bull. Mar. Sci.
54:206-227.
Lim, L.
C. 1993. Larviculture of the greasy grouper, Epinephelus tauvina
F., and the brown-marbled grouper, E. fuscoguttatus F., in
Singapore. J. World Aquacult. Soc. 24:262-274.
Mamauag, S.,
L. Penolio, P. Alino, and G. Russ.
1995. Juvenile groupers in the
Philippines: gaining insights into their recruitment patterns. Third Natl.
Symp. Mar. Sci., Philippine Assoc. Mar Sci.
Milicich, M.
J. 1992. Light-traps: a novel technique for monitoring larval supply and
replenishment of coral reef fish populations.
Unpubl. Ph.D. Thesis, Griffith University, Australia. 127 pp.
Milicich, M.
J., P. J. Doherty. 1994. Larval supply of coral reef fish
populations: magnitude and synchrony of replenishment to Lizard Island, Great
Barrier Reef. Mar. Ecol.
Prog. Ser. 110:121-134.
Milicich, M.
J., M. G. Meekan, and P. J. Doherty.
1992. Larval supply: a good
predictor of recruitment of three species of reef (Pomacentridae). Mar. Ecol. Prog. Ser. 86: 153-166.
Pascoe, P.
L. 1990. Light and the capture of marine animals. Cambridge Uni. Press.
Pauly,
D. and V. Christensen. 1995.
Primary production required to sustain global fisheries. Nature 374: 255-257.
Rawlinson, N.
J. F. 1990. Catch composition of the tuna bait-fishery of Solomon Islands and
possible impact on non-target species.
ACIAR Proc. 30: 190-211.
Sorgeloos,
P. 1994. State of the art in marine fish larviculture. World Aqua.
25: 34-37.
Wolanski,
E., P. J. Doherty, and J. H. Carleton.
1997. Directional swimming by
fish larvae determines connectivity of the Great Barrier Reef. Naturwissenshaften 84:262-268