The
Fisheries for and the Mariculture Potential of Groupers, Red Red Snappers,
Breams, and other Coral Reef Fishes in India
M. Devaraj and V.
Sriramachandra Murty[1]
Abstract
India is endowed with vast areas of coastal reefs along
the Gulf of Kutch, Kerala coast, Wadge Bank, Gulf of Mannar, Palk Bay, and the Andaman
and Lakshadweep islands. The coral
reefs constitute an important habitat for a vast range of ornamental fishes of
about 300 species. Of these, fishes of
the families Acanthuridae, Pomacentridae, Labridae, Scaridae, Chaetodontidae,
Siganidae, Holocentridae, Syngnathidae and Balistidae are important. About 40
species of groupers, a similar number of red snappers, and about 9 species of
pigface breams are known from India.
The grouper fishery comprises Epinephelus tauvina, E.
malabaricus, E. undulosus, E.
areolatus, E. merra, E. fasciatus, E. sonneratti, E.
bleekeri, E. diacanthus, E. chlorostigma, E.
caeruleopunctatus, and Promicrops lanceolatus are common in the
fishery. Among the red snappers, Lutjanus
rivulatus, L. malabaricus, L. fulviflamma, L. kasmira,
L. argentimaculatus, L. vaigiensis, L. lineolatus, L.
gibbus, and Pristipomoides typus constitute the fishery. The fishery for the pigface breams includes Lethrinus
nebulous, L. lenten, L.
miniatus, L. elongatus and L. mahsenoides. During the 1985 to 1994 period, an estimated
average annual landing of 13,616 t was obtained in India with grouper forming
44.9 %, pigface breams 31.1% and snappers 24.0%. Almost the entire catch was taken from the
0-50 m depth. The potential of these fisheries
in the Indian EEZ is estimated to be around 40,000 t in the 0-50 m depth zone
and 14,600 t in the 50-300 m depth zone.
The wide difference between the potential and the landings is due to the
grounds not being amenable to trawls and set-nets. Fishing by traps and long-lines need to be popularised for the
effective exploitation of these resources.
In the area of marine finfish culture, the country is still in the
experimental phase. Experiments on
several species Epinephelus tauvina, Siganus canaliculatus, S.
javus, Lates calcarifer, and other species were conducted. Breeding, seed production, and grow-out
technologies are yet to be developed. Very recently, lucrative nursery grounds
of grouper seed (of 60-260 mm) have been located off the southeast coast of
India and live juveniles of groupers (E. tauvina and E. malabaricus)
are exported live to Hong Kong and Singapore.
The Central Marine Fisheries Research Institute has embarked on a
programme of breeding and hatchery production of seed of groupers, snappers and
breams. A private company is currently
operating a net cage at Tuticorin in the Gulf of Mannar for captive holding and
fattening of groupers for live export in ship to Hong Kong. The company has exported in 1996 about 20
tonnes of live groupers.
Introduction
India is endowed with vast areas of coastal reefs along the
Gulf of Kutch, Kerala coast, Wadge Bank, Gulf of Mannar, Palk Bay, and the
Andaman and Lakshadweep islands. They
are distributed in the nearshore regions in the Palk Bay and the Gulf of Mannar
and the lagoons of Lakshadweep islands, in the depths of over 400-m along the
west coast and around the islands in the Andaman and Lakshadweep. Most of these areas are not suitable for
fishing with trawlers gillnets.
However, trap and long-lines are operated efficiently in these
areas. The present fishery, however, is
restricted to trawls and hooks and lines in the inshore waters and perch-traps
in the Gulf of Mannar and Palk Bay.
The fishes of the families Serranidae (groupers), Lutjanidae
(snappers), and Lethrinidae (pigface breams or emperors), together referred to
as perches in this paper, are represented by about 90 species in the Indian
seas. There is considerable potential
for the exploitation of these fish populations. The estimated potential of these resources in the Indian EEZ is
around 55,000 t. In addition to the
perches, there are around 300 species of marine fishes, which can be classified
as ornamental fishes, distributed in the vast stretches of coral reefs in the
Indian Exclusive Economic Zone (EEZ) which are not presently exploited. The commerce for live perches and marine
ornamental fishes has bright prospects based on recent developments in export
market for finfishes. It is therefore
important to accelerate the production of groupers, breams, snappers, and
marine ornamental fishes and to develop their hatchery and grow-out
technologies to promote the trade of live fishes. A review of the present
status of our knowledge on these fishes is presented here to facilitate further
action.
Overview of Marine Fish
Aquaculture
Species
Cultured
The marine fish aquaculture in India is still at the experimental
stage. Many species are presently
cultured, namely Chanos chanos, Mugil cephalus, Liza
macrolepis, Valamugil seheli, Anguilla bicolor bicolor, Etroplus
suratensis, Oreochromis mossambicus, Sillago sihama, Siganus
javus, S. canaliculatus, L. calcarifer, E. tauvina,
and a few others. The culture of the
groupers, siganids, and L. calcarifer will be considered in this review.
Production
The production from culture experiments
mainly Chanos chanos and mullets are variable (Table 1). The possible reasons for this variability
are the differences in the stocking rates and methods of culture in different
localities. For coral reef fishes, only
the grouper, Epinephelus tauvina has been cultured experimentally using
net-cages in Mandapam. The seedlings
were 16.3 mm in length and 47.5 g in weight and stocked at 100 inds/ha. The production at the end of 11 months is
288 kg.
Culture systems and their geographic
locations
The cage culture and the pen culture systems are present in India. The Central Marine Fisheries Research
Institute (CMFI) has developed facilities for experimental culture of marine
finfishes. The cage culture system was developed for groupers, rabbitfishes,
and seabass. For all the other species,
the pen culture system was developed. The inshore regions along the coast are
suitable for finfish culture using floating net cages, pen-, and cage culture
practices. Along the northeast coast,
Chilka Lake, and the Kakinada Bay are suitable.
On the southern coasts of southern Andhra
and northern Tamil Nadu, the Pulicat Lake has areas suitable for culture. The Gulf of Mannar and Palk Bay, the Vizhinjam
Bay, the estuarine backwater lakes of Kerala, the estuaries of Karnataka and
the Gulf of Kutch along the northwest coast, and the shallower lagoons in the
Andaman and Laksadweep islands are suitable for pen- and cage culture
practices.
The suitability of net-cage culture in the
Gulf of Mannar has been demonstrated. A
private entrepreneur at Tuticorin near the Vantive Island in the Gulf of Mannar
has developed recently a commercial net-cage culture system (only
holding). This firm gathers groupers
from different fishing grounds with the help of trained fishermen in the Gulf
of Mannar region. Floating net cages in
a raft anchored at 4-m depth close to this island are used for holding the live
groupers weighing from 0.5 to 15 kg.
Sardines and fresh trash fish are given as food. Efforts are in progress for a similar
venture from the Andaman waters.
Table 1.
Production in experimental culture of marine finfishes in different
culture systems
|
Species |
Stocking density (no/ha) |
Production (kg/ha) Region |
Reference |
|
Monoculture |
|||
Chanos chanos
|
6250 |
212 |
Thampi, 1960 |
|
|
4000 |
852/Mandapam |
Mohnaraj et al., 1983 |
|
|
5600[2] |
2,765-4,663/ Calicut |
Lazarus and Nandakumaran,
1987 |
|
|
2000 |
546-770/ Madras |
Nammalwar and Mohanrajm,
1991 |
|
|
7820 |
318-857/ |
Bensam and Marichamy, 1981 |
|
|
75490 |
NA[3]/Tuticorin |
Evangeline, 1967 |
|
Polyculture |
|||
|
Chanos chanos Penaeus monodon |
3,500 70,000 |
704-1,088/ Madras |
Syndararajan et al. (1979) |
|
Chanos chanos Liza macrolepis Scylla serrata |
1,450 300 617 |
1,644/ Tuticorin |
Marichamy et al. (1980) |
|
Chanos chanos Mugil cephalus Penaeus indicus |
3,500-4,982 2,428-7,364 43,200-76,383 |
498-662 |
Marichamy and Rajapackiam,
1982a, b |
|
Chanos chanos P. monodon
|
5,000 |
140-364/ Madras |
Nammalwar and Kathirvel,
1988 |
|
Chanos chanos M. cephalus
|
1,000 |
254/ Madras |
Nammalwar and Mohanraj,
1991 |
|
Chanos chanos Liza macrolepis Valamugil seheli P. monodon
|
13,000 2,000 22,000 7,000 |
1,464/ Madapam |
James et al., 1984a, b |
|
Pen culture |
|||
Chanos chanos
|
4,000 |
NA/Mandapam |
Venkataraman et al. 1985 |
|
L. macrolepis |
10,000 |
NA/Tuticorin |
Sunmugam and Bensam, 1982 |
Chanos chanos
|
40,000 |
NA/Tuticorin |
Marichamy et al., 1982 |
|
Monoculture of Grey mullets |
|||
|
Liza vagiensis |
22,000 |
135 |
James et al., 1985a |
|
Valamugil seheli |
50,000 |
387/Mandapam |
|
|
M. cephalus |
15,000 |
123 |
Nammalwar and Monhanraj, 1991 |
|
L. macrolepis |
7,000 |
387 |
Nammalwar and Monhanraj, 1991 |
|
Polyculture |
|||
M. cephalus
L. macrolepis Liza cunnesius |
2,400 5,000 |
199-752 NA/Madras |
Nammalwar and Monhanraj, 1991 |
|
Pen culture (polyculture) |
|||
L. macrolepis
P. indicus P. monodon |
50,000 |
192/ Tuticorin |
Marichamy et al., 1979 |
|
M. cephalus L. macrolepis |
40,000 40,000 |
555.9/ Tuticorin |
Marichamy et al., 1980 |
|
Pearl-spot monoculture in ponds |
|||
|
Etroplus suratensis |
5,000 |
1,000-2,000/ Kerala and Goa |
Sumitra Vijayaraghavan et al., 1981 |
|
Asian seabass pond culture |
|
|
|
|
L. calcarifer |
104-176 |
Nil/Tuticorin |
James and Marichamy, 1987 |
|
|
2,000-5,000 |
2,000/West Bengal |
Pillai and Bose, 1957 |
|
|
6,000 |
2,759/West Bengal |
|
|
Polyculture |
|||
|
L. calcarifer Oreochromis mossambicus |
444 4,444 |
217.78 213.33/Kerala |
Purushan, 1990 |
|
L. calcarifer O. mossambicus |
1,000 4,200 |
506.89 886/Kerala |
|
|
L. calcarifer O. mossambicus |
600 4,900 |
322.44 904.44 |
|
|
Cage culture |
|||
|
Epinephelus tauvina |
13 (173-354mm/80-580 g) |
19 mm/87.3 g |
James et al., 1985 |
|
E. tauvina |
100 (16.3/47.5 g) |
228kg/11 mo/ Mandpam |
Ameer Hamsa and Mohamed Kasim, 1992 |
|
E. hexagonatus |
8 (224-300mm/190-380 g) |
6mm/30 g |
|
|
Sillago sihama |
106 (63-95/2.8-6 gm) |
20.1mm/3.2g |
James et al., 1985 |
|
Siganus canaliculatus |
100 (71-91mm-4.0-10g) |
12.4 mm/2.8 g |
James et al., 1985 |
|
S. javus |
200 (67-90mm/5.2-13 g) |
16.0mm/6.2 g Mandapam |
James et al., 1985 |
Trade and
Economics
The live
fish trade is mainly for groupers although there is a great potential to export
ornamental aquarium marine fishes from the coral reefs of India. Live groupers are exported by a commercial
venture at a minimum of 10 tonnes to Hong Kong for further trading through a
chartered modern vessel (live-fish trading vessel). About 2 tonnes of live groupers, ranging from 2 to 18 kg,
comprising 3 species of groupers Epinephelus tauvina, E. malabaricus,
and E. undulosus, were exported live to Hong Kong during 1996. Live groupers are exported at the rate of Rs
150 to 200 per kg, which is about three times higher than the price in the
local markets. Ornamental aquarium
fishes is exported at a very low quantity; almost the entire trade of ornamental
fishes, valued at about Rs 100 million (Srivastava, 1994), is based on
freshwater fishes. There is vast scope
for commercial export of marine ornamental fish in view of the abundant
resources in the Indian reefs.
The
resource: coral reefs and associated fisheries
Reef distribution and associated fish species
India is endowed with vast areas of coral reefs along
the Gulf of Kutch, Kerala coast, Wadge Bank, Gulf of Mannar, Palk Bay, and the
Andaman and Lakshadweep Islands. They
are distributed in the nearshore regions in the Palk Bay and the Gulf of Mannar
and the lagoons of Lakshadweep Islands.
Coral reefs are also found down to 40 m deep and around the islands of
Lakshadweep and Andaman Islands. These
coral reefs are rich in groupers, snappers, emperors that are exploited for the
food market and about 300 species for the aquarium trade.
The coral reef fishes are exploited for the aquarium
trade from 3 general localities: Lakashadweep Islands, Andaman Islands, and the
Gulf of Mannar and Palk Bay. There are
36 islands in the Lakshadweep region that harbours a variety of aquarium fishes
(Table 2). The labrids (45 species),
damselfishes (35 species), cardinal fishes (22), groupers (21 species),
blennies (20 species), surgeon fishes (19 species), butterfly fishes (16
species), goat fishes (14 species), gobies (14 spp.), scorpion fishes (14
spp.), trigger fish (10 spp.), squirrel fish (9 spp.), and many others.
The Central Marine Fisheries Research Institute is currently engaged in a survey of the coral reef fishes that are exploited for the aquarium industry. Eight islands have been surveyed so far. The preliminary results of this study are:
·
199 species (of the 300
spp.) are common in these islands;
·
wrasses are the most dominant,
numerically contributing to 37% of the total population, followed by the
damselfishes (31.9%), parrotfishes (8.4%);
·
groupers comprise 1.2%
of the numerical abundance;
·
Kalpeni Island has the
highest number of species with 28.8% of the total population, followed by Amini
(27.6%), Kadamat (15.4%), Chetlat (9.2%); and
·
72 of the common species
in these islands (Table 2) are dominant numerically and are exploitable.
Reef Fisheries
The groupers, snappers, and emperors in the coral reefs are exploited in the inshore regions by trawls, hooks-and-lines, and perch-traps. There is no target fishing or these fishes except the hook-and-line fisheries along the Kerala and Tamil Nadu coasts and perch-trap fisheries in the Gulf of Mannar and the Palk Bay.
The yields of the coral reef fishery are high. During the period from 1984-1994, the annual
landings of groupers ranged from 2,611 tonnes in 1986 to 10,895 tonnes in 1993
and with annual average of 6,114 tonnes.
For snappers, the landings ranged from 2,228 tonnes in 1991, and 4,135
tonnes in 1988, and 3,262 tonnes on the average. For emperors, the landings during this period ranged from 1,943
tonnes in 1989 to 9,705 tonnes in 1994 with an average of 4,240 tonnes (Figure
1).
Catches of these 3 coral reef fishes are primarily
from Tamil Nadu. The maximum catch of
groupers, representing 33.5% of India’s total production, was obtained from the
Tamil Nadu coast and in Maharashtra (27.8%), Kerala (21.2%), Gujarat (9.7%),
and other States. Snappers are less
abundant along the Tamil Nadu coast (26% of total), than along the coasts of
Andhra Pradesh (24.0%), Mahrashtra (20.3%), Kerala (15.6%), Gujarat
(11.4%), and other States. The
emperors are harvested primarily from the Tamil Nadu coast with accounts for
87.5% of the India’s landings (Figure 1).
The fishery for these coral reef fishes is organised
in Tamil Nadu, particularly in the Gulf of Mannar and the Wadge Bank using
hook-and-lines, gill-nets, and perch-traps.
In the Gulf of Mannar, off Tuticorin, there are 250 to 300 country
crafts with lines and gill nets that operate in 35 to 60 m. About 85% of perches are caught by
hooks-and-lines. Serranids, which
compose about 25% of perch catches, are represented by 5 species, namely, Epinephelus
tauvina (53%), E. malabaricus (15.7%), E. diacanthus (14.1 %),
E. chlorostigma (11.1%), and E. undulosus (6.1%). Large catches are taken during July-October
(Matthew, 1995). Lutjanids form about
15% of perch catches. Lutjanus
rivulatus is the most dominant snapper (45%) and then followed by L.
argentimaculatus (26.6%), L.
malabaricus (21.2%), and Pristipomoides typus (9.6%). Sustenance fishery also occurs in Keelkarai
Village in the Gulf of Mannar.
The other location where there is a regular and
organised fishery is off Quill. The lutjanids form 80% of the catches
while serranids compose 20% of the catches during January-April every
year. The fishery uses hook and line at
50-150 m depth (Madnmohan, 1983).
Status of Exploitation of Coral Reef Associated Fishes
Perches
The
groupers (Serranidae), snappers (Lutjanidae) and pigface breams (Lethrinidae)
are among the most dominant residents fish populations in the regions. They are exploited in the inshore by trawls,
hooks and lines, and perch traps. There
is however no target-fishing for these fishes excepting the hook and line
fisheries along the Kerala and Tamil Nadu coasts and perch trap fisheries in
the Gulf of Mannar and Palk Bay.
The data on all-India production during 1985-94 showed
that the annual landings of perches were increasing (Figure 1). Landings of groupers was highest among the
perches, ranging from 2,611 t in 1986 to 10,895 t in 1993 with an annual
average of 6,114 t. For snappers, the
minimum, maximum and average during the period were 2,228 t in 1991 and 4,136 t
in 1988 and 3,262 t, respectively. With
respect the pigface breams, the annual landings varied from 1,943 t in 1989 to
9,705 t in 1994 with an average of 4,240 t (Figure 1).
These perches are caught in different states
throughout India. The maximum catches
for groupers, representing 33.5% of country's total, was obtained from
the Tamil Nadu coast followed by Maharashtra
(27.8%), Kerala (21.2%), Gujarat (9.7%), and other
States. Snappers are also more abundant
along the Tamil Nadu coast (26% of India's catch of snappers) than along the
coasts of Andhra Pradesh (24.0%), Maharashtra (20.3%), Kerala
(15.6%), Gujarat (11.4%)
and other States. The pigface breams are taken principally from the Tamil Nadu
coast that accounts for 87.5% of India's landings (Figure 1).
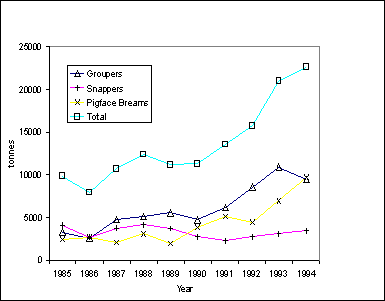
Figure 1.
Trends in the catches of selected coral reef fishes from 1985 to 1994
A large number of exploratory and experimental fishing
surveys undertaken along the Indian coasts highlight the relative and absolute
abundance of various demersal fisheries, both in spatial and temporal terms
(Bapat et al., 1982; Joseph et al., 1987; Menon and Joseph, 1969;
Ninan et al., 1984; Philip et al., 1984; Silas, 1969; Sivaprakasam,
1986; Somavanshi and Bhar, 1984). The status of present knowledge on the
distribution and abundance of demersal fishes in the Indian EEZ based on these
surveys is reviewed (James et al., 1993). The perches are exploited with trawl nets, hooks and lines, and
traps. There is however no targeted
fishing for these resources except the hook-and-line fisheries along the Kerala
and Tamil Nadu coasts. In Tamil Nadu,
there is an organised fishery for the perches in the Gulf of Mannar and the
Wadge Bank using hooks and lines, gillnets, and perch traps. In the Gulf of
Mannar off Tuticorin (8o N lat.), the bottom is rocky and rich in
coral reefs. In this region, about 250
to 300 traditional crafts operate with hooks and lines and gillnets in 35 to 60
m depths to catch perches. About 85% of
perch the catch is obtained by hooks and lines. Serranids which form about 25% of perch catch are represented by
five species of which E. tauvina is the most dominant (53%), followed by
E. malabaricus (15.7%), E. diacanthus (14.1%), E. chlorostigma
(11.1%), and E. undulosus (6.1%). Maximum catches are taken during
July-October (Mathew, 1995). Lutjanids
form about 15% of perch catch and L. rivulatus is the most dominant
species forming 43% of snappers landed, followed by L. argentimaculatus
(26.6%), L. malabaricus (21.2%), and Pristipomoides typus
(9.6%). In the Gulf of Mannar off the
Keelakarai fishing village there is a sustenance fishery by traps, which
exploit groupers and snappers along with several other coral fishes. Along the west coast, off Cochin, the
hook-and-line fishery starts around December and lasts till March (Mathew and
Venugopalan, 1990). Mechanised boats
(7.62 to 9.4 m) conduct fishing during December and January and landings of
mostly perches. Perches form over 90%
of the catch, which is dominated by E. diacanthus (19-59 cm), E.
chlorostigma (24-62 cm), E. tauvina (42-85 cm), E. bleekeri (21-64
cm), E. areolatus, and Pristipomoides typus (19-69 cm). There is a regular fishery for serranids
(20%), and lutjanids (80%) in January-April every year by traditional craft
using hooks-and-lines off Quilon (8o20'40"N lat., 77o2'5"E
long.) in the 50 to 150-m depth range (Madanmohan, 1983).
Ornamental Fishes
In the lagoons of the Lakshadweep islands
and the Andaman Islands, the Gulf of Mannar, and Palk Bay, a large number of
ornamental fishes occur among the corals and seagrasses (Table 2). However, there is very little information on
the availability, abundance, and stock of ornamental fishes. There is one reports from on Lakshadweep
(Murty et al., 1989) and another from the Andamans (Dorairaj,
1994). At present, there is no
organised exploitation of these fishes.
Table 2.
Important and common commercial species [4] in the Lakshadsweep Islands, Wandoor National Park,
and in the Gulf of Mannar and Palk Bay (4 - present)
Scientific names
|
Lakshadsweep |
Wandoor National Park |
Gulf of Mannar and Palk Bay |
|
Acanthuridae – surgeon fishes |
|||
|
1.
Acanthurus elongatus
(?) |
4 |
|
|
|
2.
A. gahhm |
|
|
4 |
|
3.
A. lineatus |
4 |
4 |
|
|
4.
A. leucosternon |
4 |
4 |
|
|
5.
A. nigricauda |
4 |
|
|
|
6.
A. nigrofuscus |
4 |
|
4 |
|
7.
A. triostegus |
4 |
4 |
|
|
8.
Ctenochaetus strigosus |
4 |
4 |
4 |
|
9.
Naso brevirostris |
4 |
|
4 |
|
10.
N. lituratus |
4 |
|
|
|
11.
N. tuberosus |
4 |
|
|
|
12.
N. unicornis |
4 |
4 |
|
|
13.
Zebrasoma veliferum |
4 |
4 |
4 |
|
Balistidae – triggerfishes |
|||
|
14.
Balistapus undulatus |
4 |
4 |
|
15.
B. viridis
(?)
|
|
4 |
|
16.
Balistoides
viridescens
|
4 |
|
|
|
17.
Odonus niger |
|
|
4 |
|
18.
Pseudobalistes flavimarginatus
|
|
|
|
|
19.
Rhinecanthus aculeatus |
4 |
|
|
|
20.
R. rectangulus |
4 |
|
|
|
Chaetodontidae – butterflyfishes |
|||
|
21.
Chaetodon annularis |
|
4 |
|
|
22.
C. aura |
4 |
4 |
4 |
|
23.
C. citrenellus |
4 |
|
|
|
24.
C. collare |
|
|
4 |
|
25.
C. falcula |
4 |
4 |
|
|
26.
C. lineolatus |
|
4 |
|
|
27.
C. lunula |
4 |
|
|
|
28.
C. melannotus |
|
|
4 |
|
29.
C. octofasciatus |
|
|
4 |
|
30.
C. plebeius |
|
|
4 |
|
31.
C. trifasciatus |
4 |
4 |
4 |
32.
C.
vagabundus
|
|
4 |
|
|
33.
C. xanthocephalus |
|
|
4 |
|
34.
Heniochus acuminatus |
4 |
4 |
4 |
|
35.
Megaprotodon
striangulus (?) |
4 |
|
|
|
Ephippidae – batfishes |
|||
|
36.
Platax orbicularis |
|
4 |
4 |
|
37.
P. teira |
|
|
4 |
|
Holocentridae – squirrelfishes |
|||
|
38.
Myripristis adusta |
4 |
|
|
|
39.
M. murdjan |
4 |
|
|
|
40.
Sargocentron caudimaculatum |
4 |
|
|
|
41.
S. diadema |
4 |
|
4 |
|
42.
S. punctatissimum |
4 |
|
|
43.
S. rubrum
|
|
4 |
4 |
|
44.
S. spinifer |
4 |
|
|
|
45.
S. violaceum |
4 |
|
|
|
Labridae – wrasses |
|||
|
46.
Anampses caeruleopunctatus |
|
4 |
|
|
47.
Cheilinus chlorourus |
|
|
4 |
|
48.
C. trilobatus |
4 |
|
4 |
|
49.
C. undulatus |
|
|
4 |
|
50.
Cheilio inermis |
|
|
4 |
|
51.
Epibulus insidiator |
|
|
4 |
|
52.
Gomphosus caeruleus |
4 |
4 |
|
|
53.
G. varius |
4 |
4 |
|
|
54.
Halichoeres
centiquadrus |
4 |
|
|
|
55.
H. leucurus |
|
|
4 |
|
56.
H. marginatus |
4 |
|
|
|
57.
H. nigriscens (?) |
|
|
4 |
|
58.
H. scapularis |
4 |
|
|
|
59.
Stethojulis albovittata |
4 |
|
|
|
60.
S. balteata |
|
|
4 |
|
61.
S. interrupta |
|
|
4 |
|
62.
S. phaekadopleura (?) |
|
|
4 |
|
63.
S. strigiventer |
4 |
|
|
|
64.
S. trilineata |
4 |
|
|
|
65.
Thalassoma hardwicke |
4 |
4 |
4 |
|
Monacanthidae - filefishes |
|
|
|
|
66.
Aluterus scriptus |
|
|
4 |
67.
Paramonacanthus
choirocephalis
|
|
|
4 |
|
68.
Mulloidichthys flavolineatus |
4 |
|
4 |
|
69.
Parupeneus barberinus |
4 |
|
|
|
70.
P. bifasciatus |
4 |
|
|
|
71.
P. Forskåli |
4 |
|
|
|
72.
P. indicus |
|
|
4 |
|
73.
P. macronema |
4 |
|
|
|
74.
P. pleurospilus (?) |
|
|
4 |
|
75.
Upeneus displurus (?) |
|
|
4 |
|
Mullidae – goatfishes |
|
|
|
|
76.
U. japonicus |
|
|
4 |
|
77.
U. luzonius |
|
|
4 |
|
78.
U. oligospilus (?) |
|
|
4 |
|
79.
U. sulphureus |
|
|
4 |
|
80.
U. sundaicus |
|
|
4 |
|
81.
U. tragula |
|
|
4 |
|
82.
U. vittatus |
|
|
4 |
|
Ostraciidae – boxfishes |
|||
83.
Lactoria
cornuta
|
|
|
4 |
84.
Ostracion
nasus
|
|
|
4 |
|
Pomacanthidae – angelfishes |
|
|
|
85.
Centropyge multispinis
|
4 |
|
|
|
86.
Pomacanthus annularis
(?) |
|
|
4 |
|
87.
P. imperator |
4 |
4 |
|
|
88.
Abudefduf biocellatus
(?) |
|
|
4 |
|
89.
A. saxatilis |
|
4 |
4 |
|
90.
A. sexfasciatus |
4 |
|
|
|
91.
A. semptemfasciatus |
|
|
4 |
|
92.
A. xanthozona (?) |
4 |
|
|
|
93.
Amphiprion percula |
|
4 |
|
|
94.
A. sebae |
|
|
4 |
|
95.
Chromis caerulea |
4 |
4 |
|
|
96.
C. chrysura |
4 |
|
|
|
97.
C. dimidiata |
|
4 |
|
|
98.
Chrysiptera glauca |
4 |
|
|
|
99.
Dascyllus aruanus |
4 |
4 |
|
|
100.
D. reticulatus |
4 |
|
|
|
101.
Plectroglyphidodon dickii |
4 |
|
|
|
102.
P. lacrymatus |
4 |
|
|
|
103.
Pomacentrus
albicaudatus |
4 |
|
|
|
104.
P. brachialis |
4 |
|
|
|
105.
P. trilineata |
4 |
|
|
|
106.
Stegastes nigricans |
4 |
|
|
|
Scaridae – parrotfishes |
|||
107.
Callyodon
bataviensis (?)
|
4 |
|
|
108.
C.
dussumieri (?)
|
|
|
4 |
109.
C. scaber
(?)
|
4 |
|
|
110.
C. taeniurus
(?)
|
4 |
|
|
111.
Calotomus
spinidens
|
4 |
|
|
112.
Chlororus
sordidus
|
4 |
4 |
|
|
113.
Leptoscarus vaigiensis |
4 |
|
4 |
114.
Scarus
ghobban
|
|
|
4 |
|
Scorpaenidae – scorpion
fishes |
|||
|
115.
Pterois volitans |
|
4 |
4 |
116.
P. miles
|
|
|
4 |
|
Serranidae – groupers |
|||
|
117.
Cephalopholis argus |
4 |
4 |
|
|
118.
C. boenak |
|
|
4 |
|
119.
C. leopardus |
|
4 |
|
|
120.
C. miniata |
|
4 |
4 |
|
121.
Epinephelus merra |
4 |
4 |
4 |
|
122.
E. hexagonatus |
4 |
|
|
|
123.
E. undulosus |
|
|
4 |
|
Siganidae – rabbitfishes |
|||
124.
Siganus
canaliculatus
|
|
|
4 |
|
125.
S. javus |
|
|
4 |
126.
S. spinus
|
|
4 |
|
|
127.
S. stellatus |
|
4 |
|
|
128.
S. virgatus |
|
4 |
|
|
Syngnathidae – seahorses, pipefishes |
|||
129.
Hippocampus
kuda
|
|
4 |
|
130.
Migrognathus
brevirostris
|
|
|
4 |
131.
Microphis
brachyurus
|
|
4 |
|
|
132.
Syngnathoides
biaculeatus |
|
|
4 |
|
Tetraodontidae – pufferfishes |
|||
|
133.
Arothron immaculatus |
|
|
|
|
134.
A. hispidus |
|
|
|
|
135.
A. reticularis |
|
|
|
|
136.
Canthigaster
margaritata |
4 |
|
4 |
|
Tetrarogidae – cobbler |
|||
|
137.
Gymnapistes dracaena (?) |
|
|
4 |
|
Zanclidae – moorish idol |
|||
|
138.
Zanclus cornutus |
4 |
|
4 |
|
Total |
81 |
37 |
65 |
Distribution
Lakshadweep Islands - There are 36 islands
in this region and the lagoons and reef flats harbour rich ornamental fish
fauna. Of over 600 species of marine fishes
reported from this region, about 300 species belonging to about 35 families are
known for their attractive colours and shapes.
Among them, the wrasses (Labridae), constitute the largest group with 45
species followed by damselfishes (35 species), cardinal fishes (22 species),
groupers (21 species), blennies (20 species), surgeon- or unicorn fishes (19
species), butterfly fishes (16 species), goatfishes (14 species), gobies (14
species), scorpion fishes (14 species), triggerfish (10 species), squirrel
fishes (9 species), and others.
The Central Marine Fisheries Research
Institute is currently engaged in the survey of ornamental fish resources of
the Lakshadweep. The tentative results
of this study are outlined below:
·
Of the total of over 300
species known, the surgeon fishes, parrot fishes, puffer fishes, butterfly
fishes, squirrel fishes, wrasses, trigger fishes, file fishes, goat fishes,
angel fishes, damsel fishes, groupers, and moorish idols, comprising 199
species are common in these islands;
·
Of the 199 species, 72
species (Table 2) are dominant numerically and offer considerable scope for
exploitation and export;
·
Among them the wrasses
are the most dominant numerically constituting 37% of the total population,
followed by the damselfishes (31.9%);
·
parrotfishes (8.4%),
goat fishes (8.2%), squirrel fishes (4.7%), surgeon fishes (4.6%), butterfly
fishes (2.1%), groupers (1.2%), trigger fishes (0.8%), puffer fishes (0.6%),
moorish idols (0.4%), and angel fishes (0.1%); and
·
Of the eight islands
surveyed so far, Kalpeni is the richest in the above groups of fishes with
28.8% of the total population followed by Amini (27.6%), Kadamat (15.4%),
Chetlat (9.2%), Kavaratti (7.4%), Agatti (6.3%), Kiltan (3.2%), and Bitra
(2.2%).
Andaman Group of islands - These islands offer a variety of habitats such as
rocky coasts with tidal pools, extensive backwaters, bays and mudflats that
provide potential areas for the exploitation of rich and varied marine
fishes. About 150 species of ornamental
fishes are known to be available in these islands. They include the squirrelfishes, scorpionfishes, groupers,
butterflyfishes, batfishes, angelfishes, damselfishes, wrasses, blennies,
surgeonfishes, rabbitfishes, triggerfishes, boxfishes, puffers, and other
species. In the marine national park,
Wandoor, Andamans, a survey conducted recently showed that the angelfishes were
the most abundant forming 32% of the total population followed by snappers and
fusiliers (19%), surgeonfishes (18%), coral fishes (12%), spinefoot (4%),
wrasses (3%), and others (2%).
Majority of these species is of ornamental value (Table 2).
Gulf of Mannar and Palk Bay - This region has several islands with extensive
fringing coral reefs. About 70 species
of ornamental fishes belonging to about 19 families (Table 2) are known from
this region. Butterflyfishes, wrasses, damselfishes, rabbitfishes, and
scorpionfishes are dominant.
Fishery for
ornamental fishes
In India, marine ornamental fishes are
least exploited at present but a variety of gears can be used for its
exploitation. Gears like lift-nets,
seines, scoop-nets, and traps can be used for the exploitation of ornamental
fishes. A trap, measuring 4'x2'x2', is
an effective and ideal gear for 4'x2'x2', is an effective and ideal gear for
the collection of ornamental fishes.
These traps are immersed with baits of live mussels and retrieved the
next morning. Each trap yields an
average of 20-25 fishes per day. Ten
such cages operating as one unit can yield 200 to 250 fishes a day without
causing any damage to the coral reef ecosystem.
Aquaculture Systems
and Management
At present, marine finfish culture is not
practised even on a small scale in India.
Studies in induced breeding and seed production of various species of
grey mullets such as Mugil cephalus, Liza parsia, and Liza macrolepis
were pioneered by Chaudhuri et al. (1977), Sebastian and Nair (1975),
Alikunhi et al. (1971), and Kowtal and Gupta (1983). Dorairaj et al. (1980) obtained only
limited success in induced breeding of the marine eel Anguilla bicolor. However, dietary items for rearing larvae of
marine fishes are already available.
The Central Marine Fisheries Research Institute has achieved a
breakthrough in the large-scale production of live feed organisms such as
different species of microalgae, rotifer, Artemia, Moina, and Daphnia
which constitute the major dietary items for rearing the larvae.
Aquaculture at present involves mainly the
grow-out of milkfish and groupers.
Grow-out experiments carried out so far show that the milkfish can be
stocked at a density of 5,000/ha. The
stocking density of grouper is around 5,000 to 7,000/ha at present. Small individuals of Tilapia are
released in the same pond to serve as forage fish for the groupers. However, the production potential of various
cultivable marine finfish, such as the mullets is high, ranging from 76 kg/ha
to 540 kg/ha (unpub. data).
Constraints of Culture
There many constraints in the culture of
marine finfishes. The availability and
procurement of broodstock from the wild is still a serious constraint. Hatchery
development to support of marine finfish farming has not yet taken place. Financial support from government agencies
is absent or minimum for finfish-farming projects. Finfish farming does not seem to be as profitable as
shrimp-farming.
Environmental Issues and Management
Issues related to exploitation
In
India, marine ornamental fishes are known to be abundant in coral reef areas in
shallow waters in the Gulf of Mannar and Palk Bay, around the Andaman Islands
and the lagoons and the reef flats of the Lakshadweep Islands. Among these areas, the Lakshadweep region is
the most important in regard to the abundance of ornamental fishes and in the
diversity of species. This region is
very shallow and is vulnerable to environmental degradation by human
activities. Hence, commercial
exploitation is likely to result not only in quick overexploitation but also in
the destruction of the coral reef environment.
Any project for the exploitation and export of marine ornamental fishes
should consider the following issues (Murty, 1996):
·
Destruction of
environment - The clownfish (Amphiprion
spp.) are known to be symbiotic with sea anemones while other fishes are
closely associated with coral colonies.
Some of the gears used to exploit these tend to destroy the corals. Thus, proper measures should be in place to
monitor exploitation and protection of the environment. The conservation measures should be
implemented from the very beginning to contain or stop the adverse affects.
·
Over-exploitation - Only non-destructive methods of exploitation, like
the traps, should be permitted. As some fishes do not enter traps, net
enclosures could be permitted. Though
the marine ornamental fishes are more abundant in the island ecosystems, their
exploitation and export on a small-scale may have to be initiated in the
mainland coast particularly the Gulf of Mannar and Palk Bay and extended to the
islands later.
·
Monitoring the
exploitation and export - In view of the
lucrative market for marine ornamental fishes, several firms are likely to
enter the trade and export through many centres to different destinations. For the purpose of monitoring the
exploitation and export, a single agency may be created to monitor and oversee
the trade of ornamental fishes.
Transportation, both domestic and abroad may be channelled through this
agency. Data on the location of
fishing, species, number, and size of fish may be recorded. The allowable catch may be fixed for each
location and ecosystem so as to keep the exploitation under control.
·
Breeding and culture - As the demand for ornamental fishes is increasing
rapidly, the wild stocks may suffer
overexploitation. Therefore the
technology of breeding, seed production, and culture should be developed and
hatcheries and grow-outs established at Vizhinjam, Tuticorin, and Mandapam in
the Gulf of Mannar and Palk Bay coasts.
·
Sanctuaries - One or two lagoons of the Lakshadweep islands,
particularly the Bangaram which is open for tourists, may be identified as
sanctuaries for coral reef fishes. The
marine park in the Gulf of Mannar and the Wandoor National Park in the Andaman
Islands should receive consistent support to help conserve their biodiversity.
Information on impact of wild seed
collection
The Central Marine Fisheries Research Institute has organised a programme in the 1980's to assess the seed resources of commercially important marine finfishes all along the Indian coast. In this programme, the occurrence and abundance of wild seed in relation to the environmental factors were studied. Milkfish has two peak seasons during April-June and September-October. Among the coral reef fishes, groupers are abundant during September-December in the coastal Gulf of Mannar.
Aquaculture
and environmental management
Aquaculture
vis-à-vis Coastal Regulations Zone rules and regulations - The aquaculture
policy recently declared by the Government of India ensures protection of the
coastal environment. The policy gives
extensive guidelines on how the coastal environment should be protected from
possible pollution or other damages consequent to the setting up of aquaculture
farms. Under the Coastal Regulation
Zone (CRZ), any type of construction within the 500 m landward from the High
Tide Line (HTL) is prohibited. The
aquaculture policy demands the protection of the integrity of the shore as a
condition in constructing a farm.
Hatcheries are also permitted in the CRZ possibly with inferred
provision for pollution control measures.
The relevant extracts from the concerned Acts, Rules, and Notifications
are stated below:
·
Environment (Protection)
Act 1986, under Section 3(1) and 3(2)(v) and Rule 5(3)d of Environment
(Protection) Rules, 1986 – The Notification on 19 February 1991 declares the
coastal stretches as Coastal Regulation Zone (CRZ) and regulates activities in
the CRZ. The 1986 rules apply to the
coastal stretches of seas, bays, estuaries, creeks, rivers and backwaters that
are influenced by tidal action up to 500 metres from the HTL landward. The 1991 Notification imposes restrictions
on industries, operations and processes in the CRZ.
·
2(iii) of the 1991
Notification - Setting up and expansion of fish processing units including
warehouse are prohibited. This
restriction seems too harsh and unreasonable.
However, hatcheries, that require inevitably a waterfront, are
permissible.
·
Para. 3(1) of
Notification - Clearance for certain activities within the Coastal Regulation
Zone Clearance shall be given for any activity only if it requires waterfront
and foreshore activities. This implies
that if the clearance is obtained from the Ministry of Environment,
aquaculture/hatcheries can be set up.
However, there is no mention of aquaculture in the list of allowable
activities, such as agriculture, horticulture, and salt manufacture, within
category III (200 to 300 m) of the CRZ.
Construction of hotels/beach resorts are allowed with prior approval.
Thus,
the essence of the Notification is that the coastal ecosystem should not be
damaged. This is echoed in the
Aquaculture (Regulation) Act 1995 of the State of Tamil Nadu that permits
aquaculture activity within the 500 m CRZ, albeit with some environmental
safeguards.
Marketing and economic aspects
The economic aspects of marine finfish farming need to be standardised on the basis of farming practices for different zones. The fry and fingerlings of some species of groupers occur in large quantities close to estuaries, offering good scope to promote live export. The southeast coast of India has been identified as a potential ground for the collection of a variety of groupers, snappers, and pigface breams and the Gulf of Mannar, Lakshadweep and Andamans for ornamental fishes. The standardisation of farming practices will improve production and increase the export of live groupers and ornamental fishes at premium prices to many countries.
Constraints to grouper and other coral reef fish aquaculture
The main constraint in the aquaculture of groupers and other coral reef fishes is technical in nature. The technology of breeding and hatchery production is yet to be developed and standardised. Grow-out techniques need to be developed and perfected although a beginning has been made. Technology transfer and extension methods need to be strengthened.
Recommendations
To
develop marine fin-fish culture technologies, there is need for:
·
training scientists in
breeding and hatchery development and management;
·
carrying out research in
physiology, biochemistry, and biotechnology pertaining to reproduction and
hatchery development; and
·
developing policy on
land and water use for coastal mariculture.
Literature Cited
Ameer
Hamsa, K.M.S. and H. Mohamed Kasim. 1992.
Growth and production potential
of young grouper, Epinephelus tauvina (Forskål) reared in fixed net
cages. J. Mar. Biol. Ass. India 34 (1
& 2): 271-276.
Bapat, S. V., V. M. Deshmukh,
B. Krishnamoorthi, C. Muthiah, P. V. Kagwade, C. P. Ramamirtham, K. J. Mathew,
S. Krishnapillai, and C. Mukundan. 1982. Fisheries resources of the exclusive
economic zone of the northwest coast of India. Bull. Cent. Mar. Fish. Res.
Inst. 33: 80 pp.
Bensam, P.
1985. Some engineering problems
in the construction and maintenance of marine culture ponds at Mandapam. Indian J. Fish. 32(4):417-431.
Bensam,
P. 1993. Prospects of farming groupers in India. Mar. Fish. Infor. Serv. T & E Ser. 123:1-4.
Dorairaj, K. 1994.
Study on the marine fauna in marine national park, Wandoor, South
Andamans. Report submitted to the State
Council of Science and Technology, A & N Administration, Port Blair, CARI,
Port Blair, 166 pp.
Evangeline, G. 1967. Chanos chanos culture at the
brackishwater fish farm, Adyar, Madras.
J. Fish. 3: 68-115.
Gopinath, K.
1954. A note on some deep-sea fishing
experiments off the South Western Coast of India. Indian J. Fish. 1:163-216.
James, P. S. B. R. 1985.
A review of marine finfish culture in India, its problems and
prospects. Proc. Symp. Coastal
Aquacultur. Mar. Biol. Ass. India
3:718-731.
James, P. S. B. R, R. Soundararajan, and J. X. Rodrigo. 1985.
Preliminary studies on culture of finfishes in cages in the coastal
waters of Palk Bay at Mandapam. Proc.
Symp. Coastal Aquaculture, Mar. Biol.
Ass. India, Jan 12-16, 1979, Cochin. No. 3: 910-915.
James, P. S. B. R., K. Alagarswami, K. V. Narayana Rao, M. S. Muthu, M.
S. Rajagopalan, K. Alagaraja, and C.
Mukundan. 1987. Potential Marine Fishery Resources of
India. CMFRI Spl. Pub. 30:44-74.
James,
P. S. B. R., G. Mohanraj, V. S. Rangaswamy, and A. Raju. 1985.
Preliminary experiments on the culture of grey mullets at Mandapam. Proc. Symp. Coastal Aquaculture. Mar. Biol. Ass. India, Jan 12-16, 1979,
Cochin. 3: 791-796.
James,
P. S. B. R., V. Gandhi, G. Mohanraj, A. Raju, and V. S. Rangaswamy. 1985.
Monoculture of grey mullets in coastal salt water ponds at
Mandapam. Indian J. Fish.
32(2):174-184.
James,
P. S. B. R., R. Soundararajan, and J. X. Rodrigo. 1985. Preliminary studies
on culture of finfishes in cages in the coastal waters of Palk Bay at
Mandapam. Proc. Symp. Coastal
Aquaculture, Mar. Biol. Ass. India, Jan. 12-16,1979, Cochin, 3: 910-915.
James,
P. S. B. R. and R. Marichamy.
1985. Status of Seabass culture
in India. In: J. W. Gopland and D. L. Grey.
ACIAR Proceedings 20:127-131.
James,
P. S. B. R., V. Sriramachandra Murty, and P. Nammalwar. 1993.
Studies on the fishery, biology, ecology, and cultivation of groupers
and snappers along the Indian coast - Exploitation and management. International Workshop
on Tropical Groupers and
Snappers, 26 - 29, October, 1993,
Campeche, Mexico.
Jayasankar,
P. 1990. On the seasonal hooks and line
fishery at Pamban near Mandapam. Mar.
Fish. Infor. Serv. T & E. Ser.
105:13-14.
Joel,
J. J. and I. P. Ebenezer. 1991.
Note on the giant perch caught off
Kanyakumari. Mar. Fish.
Infor. Serv. T &. E Ser.,
108:15.
Joseph, K. M., P. Sulochanan, M. E. John, V. S. Somavanshi, K. N. V.
Nair, and A. Joseph. 1987. Demersal fishery resources of Wadge
Bank. Bull. Fish. Surv. India, 12:1-52.
Lazarus, S. and K. Nandakumaran.
1987. Culture of milkfish in
polythene film-lined ponds. Mar. Fish.
Infor. Serv. T & E Ser. 76: 9-12.
Madanmohan,
x. 1983. Kalava Fisheries of
Pulluvila Village. Indian J. Fish. 30:135-142.
Marichamy, R. and S. Rajapackiam.
1982. Farming the coastal
land at Tuticorin. Mar. Fish. Infor. Serv. T & E Ser.
47:13-15.
Marichamy,
R. and S. Rajapackiam. 1982.
The culture of
milkfish, mullet, and prawns in
an experimental marine fish farm at Tuticorin.
Proc. Symp. Coastal Aquaculture.
Cochin 1: 256-265.
Marichamy, R.,
G. Venkataraman, K. M., S. Ameer
Hamsa, P. Nammalwar, P. Bensam, and S. Shanmugham 1979. Culture of fishes in cages and pens along the
coastal waters of India. Proc. Int. Workshop. Cage Pen Cult.,
SEAFDEC, Phils. 41-45 pp.
Marichamy,
R., S.
Shunmugam, and M. E.
Rajapandian. 1980. Polyculture experiments in coastal waters at
Tuticorin. Proc. Sem. Coastal Inland
Fish Cult. Tamil Nadu. 241-250.
Marichamy,
R., S. Shanmugam, and S. Rajapackiam. 1980. Polyculture experiment in Coastal waters at Tuticorin. Proc. Seminar Coastal Inland Fish Cult. in Tamil Nadu. pp.
241-250.
Mathew,
G. and
K. M. Venugopal. 1990. Hooks and line fishery for Kalava at
Cochin. Indian J. Fish. 37:347-355.
Mathew , G. 1994. On the perch fishery of Tuticorin during
1978-80. J. Mar. Biol. Ass. India.
36(1&2):28-33.
Menon, M. D. and K. M.
Joseph. 1969. Development of "Kalava" (rockcod) fishing of southwest
coast of India - A prospectus. Seafood
Export J. 1(2):7-28.
Menon, M. D., C. P. Varghese, and C. Haridas. 1977. Development of trap
fishing for rockcod off southwest coast of India. Seafood Export J. 9(1): 5-14.
Mohanraj, G., A. Raju, V. Gandhi, and V. S. Rengasamy. 1983.
Fish culture in marine farm at Mandapam. Mar. Fish. Infor. Serv. T & E Ser. 48: 1-8.
Murty,
V.S. 1969. Catalogue of fishes
(excluding from the Laccadives) in the reference collections of the Central
Marine Fisheries Research Institute. Bull. Cent. Mar. Fish. Res. Inst. 10: 36
pp.
Murty, V. S.
1996. Marine ornamental fishes
of India. pp. 23-34. Proc. Sem.
Fisheries Multibillion Dollar Industry Aquaculture Foundation of India & Fisheries Technocrats Forum, Madras,
246 pp.
Murty, V. S., M. Kumaran, and R. S. Lalmohan.
1989. Resources of ornamental
fishes. In: Marine Living Resources of Union Territory of Lakshadweep, an
indicative survey with suggestions for development. Bull. Cent. Mar. Fish. Res. Inst. 43: 4 54.
Nammalwar, P. 1995. Present status of marine finfish culture development in India. Proceedings of
National Conference on Sustainable Aquaculture 5-6, April, Anna
University, Madras. pp. 29-46.
Nammalwar,
P. and M. Kathirvel. 1988.
Preliminary experiments on monoculture of Chanos chains (Forskål)
and its polyculture with Pennies monodon Fabrics. Indian J. Fish. 35(3):197-204.
Nammalwar, P.
and G. Mohanraj. 1990. A review of marine finfish culture research
in India. Bull. Cent. Mar. Fish. Res.
Inst. 44, Part 2: 373-383.
Nammalwar, P. and
G. Mohanraj. 1990.
Present status on induced breeding of marine finfishes in India. Bull. Cent.
Mar. Fish. Res. Inst. 44, Part 2: 383-393.
Nammalwar, P. and G. Mohanraj.
1991. Farm development and
management for marine finfish culture at Muttukadu near Madras. In: Aquaculture productivity (Ed). V. R.
P. Sinha and H. C, Srivastava. Oxford
and IBH Publishing Co., Pvt. Ltd., New
Delhi, 549-563.
Nammalwar,
P., R. Marichamy, G. Mohanraj, V. S.
Rengasamy, A. Raju, and V. Gandhi.
1994. A review of the systems
for culture of marine finfishes in India.
pp. 231-244. In Proceedings of the National Symposium on
Aquaculture for 2000 A. D., 26-27, Nov.
1994, Madurai Kamaraj University, Madurai, Tamil Nadu.
Nammalwar, P., R. Marichamy, A. Raju, P. Jayasankar, M. R.
Arputharaj, C. Kasinathan, and S.
Palanichamy. (Abs.) 1996. On collection, transport, and maintenance of
Asian seabass, Lates calcarifer (Bloch). Fourth Asian Fisheries Forum, 24-28 Nov. 1996, Cochin.
Ninan,
T. V., S. P. Basu, and P. K. Bhargava.
1984. Observations on the
demersal fishery resources along Andhra Pradesh coast. Bull. Fish. Surv. India. 13:18-22.
Oomen,V.
P. 1976. An account of the fishery and biology of velameen, Pristipomoides
argyrogrammicus (Valenciennes). J. Mar. Biol. Ass. India. 18:469-475.
Oomen, V. P. 1989.
A critical study on the exploitation of fishery resources by the
Integrated Fisheries Project. Bull. IFP. 12:106 pp.
Pillay,
T. V. R. and B. Bose. 1957. Observations on the culture of brackishwater
fishes in paddy fields in west Bengal, India. Proc. Indo-Pac. Fish. Coun.
187-192.
Pillai, S. H., P. K.
Ghosh, T. Rajyalakshmi, and A. K. Roy.
1985. Observations on growth,
survival and production of grey mullets, Mugil cephalus (Linnaeus),
Liza parsia (Hamilton) and Liza tade (Forskål) in a coastal low
saline polyculture pond. Proc. Symp.
Coastal Aquaculture, Mar. Biol. Ass. India, 12-16 Jan, 1979, Cochin. 3:776-781.
Pillai, P. K .M.
1991. On the landing of giant rockcod at Cuddalore. Mar. Fish. Infor. Serv. T & E. Ser. 108:16.
Philip, K. P., B.
Premchand, G. K. Avadh, and P. J. Joseph.
1984. A note on the deep-sea
demersal resources off Karnataka-north Kerala coast. Bull. Fish. Surv. India. 13: 23-32.
Premalatha,
P. 1989. Fishery and biology of
rockcods (Order: Perciformes) from the southwest coast of India. Indian J. Fish. 36(4):285-291.
Purushan,
K. S. 1990. On the experimental
mariculture of the seabass, L. calcarifer (Bloch) with Oreochromis
mossambicus (Peters). Indian J.
Fish. 37(1):25-30.
Rangarajan, K. 1972a.
Food and feeding habits of the snapper, Lutjanus kasmira
(Forskål) from the Andaman Sea. Indian J. Fish. 17: 43-52.
Rangarajan,
K. 1972b. Maturity and spawning of the snapper Lutjanus kasmira
(Forskål) from the Andaman Sea. Indian
J. Fish. 18:114-125 (1971).
Rengaswamy,
V. S., R. Marichamy, S. Rajapackiam, and D. Sundararajan. 1996. Resources
collection and transportation of groupers for farming. (Abs.) Fourth Asian Fisheries Forum, Cochin, 24-28
Nov. 1996.
Silas,
E. G. 1969. Exploratory fishing by
R.V.Varuna. Bull. Cent. Mar. Fish. Res.
Inst. 12:86 pp.
Sivaprakasam, T.
E. 1986. A study of the demersal resources of the Wadge Bank and Gulf of
Mannar. Bull. Fish. Surv. India. 15:1-37.
Somvanshi, V. S. and P. K. Bhar.
1984. A note on demersal fishery
resources survey of Gulf of Mannar.
Bull. Fish. Surv. India 13:12-17.
Srivastava,
L. S. 1994. Ornamental fish, New Export opportunities. Yojana, November 15:22.
Sulochanan, P. and
M. E. John. 1988. Offshore, deepsea and oceanic fishery
resources of Kerala coast. Bull. Fish.
Surv. India. 16: 27-48.
Sundararajan, D., S.
Victor Chandra Bose, and V. Venketesan.
1979. Brackishwater fish and
prawn culture at Santhome Brackishwater Fish farm. Madras. J. Inland Fish. Soc. India, 11 :109-116.
Sumitra Vijayaraghavan, L. Krishnakumari,
V. G. Gopinathan, and R. M. Dhawan.
1981. Aquaculture of pearl spot,
Etroplus suratensis, in an estuarine pond: Environmental characteristics
primary production, growth and cost-benefit ratio. Indian J. Mar. Sci. 10:82-89.
Tampi, P. R. S. 1960. Utilization of saline mudflats for fish
culture. An experiment in marine fish
farming. Indian J. Fish. 7(1):137-146.
Venkataraman, G., K.M.S. Ameer Hamsa and P. Nammalwar.
1985. Fish culture in pens in the Gulf
of Mannar, India. Proc. Symp. Coastal
Aquaculture, Mar. Biol. Ass. India, 12-16
Jan. 1979, Cochin. 3:951-954.
Vivekanandan, E., C. Gopal, S. Shanmugam, H. K. Dhokia and B. P. Thumber. 1990. Industrial Fisheries off Saurashtra coast based on exploratory survey during 1985-88. Mar. Fish. Infor. Serv. T. & E. Ser. 103:1-5.