Nutritional research in Australia to improve pelleted
diets for grow-out barramundi Lates calcarifer (Bloch)
Kevin C Williams[1] and
Chris Barlow[2]
Abstract
The farming of Asian seabass or barramundi, Lates calcarifer,
is an emerging aquaculture industry in Australia with expected production to
exceed 500 t in 1995/96. In Australia, barramundi
are fed exclusively on extruded dry diets.
Since 1992, an intense research program supported by the Australian
Fisheries Research and Development Corporation has examined the nutritional
requirements of grow-out barramundi and assessed the nutritive value of
locally available protein meals.
Optimal feeding practices have been defined for juvenile barramundi
held at water temperatures varying incrementally from 20 to 29°C (the range
normally experienced on Australian farms).
Evaluation of alternative feed ingredients has shown that animal
by-product meals such as meat meal and poultry offal meal are highly palatable
to barramundi and as well-digested as fishmeal. Vegetable protein meals such as soybean,
canola and lupin are less well- digested and not well-liked but can be used
cost-effectively for the partial replacement of fishmeal. Increasing the dietary concentration of a
reference protein incrementally from 29 to 57% crude protein (CP) caused food
intake and food conversion to decrease and improve curvilinearly, respectively,
such that growth rate exhibited a bent-stick response, increasing linearly up
to about 46% CP. From these studies,
the optimum protein to digestible energy (DE) ratio of the diet was estimated
to be about 24-25 mg CP/kJDE. The essential fatty acid requirements (as the sum
of eicosapentaenoic and docosahexaenoic acids) were found to vary with water
temperature from ≈5 mg/g at 20°C to 18 mg/g at 29°C. Under laboratory and commercial farm
conditions, diets formulated entirely from terrestrial feed ingredients (except
for a low inclusion of fish oil to provide essential n-3 fatty acids) have
resulted in as good, if not better, barramundi productivity as
conventional diets based on fishmeal.
Using trained taste panels, the
eating quality of the fish reared on these nil-fishmeal diets has been the same
as for conventional diets.
Introduction
In Australia, Asian seabass or barramundi, Lates
calcarifer, is a highly-priced recreational and capture fishery. An emerging aquaculture industry is expected
to produce more than 500 t of fish in 1995/96 worth AUD $5 M (Figure 1). Most farmed barramundi are sold as
plate size (400 to 500 g) whole fish destined for the restaurant trade although
there is some interest in growing fish to a larger size (2 to 3 kg) for the
fillet trade.
A major impediment to continued expansion of
barramundi farming is the high cost of feeding since food comprises 40-50% of
on-farm costs. In Australia, all farmed
barramundi are grown-out on pelleted (extruded) dry diets which are expensive
(AUD $1,200 to $1,500/t). Feed cost is
high as diets currently contain large mounts of expensive,imported fishmeal and
because of lack of information on the fish's nutrient requirements hinders the
development of cost-effective feeds and feeding strategies.
Research to define the nutrient requirements of grow-out fish and shrimp and to assess the suitability of terrestrial protein sources, as cheaper alternatives to fishmeal, is a major priority for Australian aquaculture. This is being addressed in a nationally co-ordinated research program administered by the Australian Fisheries Research and Development Corporation. A large team of aquaculturists from Commonwealth, State and University research institutions and private industry is working collaboratively to develop improved and more cost-effective grow-out diets for barramundi, shrimp (Penaeus monodon), silver perch (Bidyanus bidyanus), and Atlantic salmon (Salmo salar). This paper reviews our work with barramundi to determine their requirements for critically important nutrients and to assess the suitability of locally available terrestrial feedstuffs as cheaper alternatives to fishmeal in manufactured diets.
Effect of water
temperature on food intake and growth
In Australia, barramundi are grown-out
typically in cages suspended in estuarine water or in fresh-brackish water in
earthen ponds. In areas where barramundi
are farmed, water temperature varies seasonally between 20°C and
29-30°C. Because water temperature is
known to have a profound effect on food intake of aquatic animals (Braaten,
1978; Steffens, 1989; Talbot, 1993), studies to define optimal feeding
practices for juvenile (≈30 to 300 g) barramundi examined the
effects of water temperature, feeding frequency and fish size (weight). Food
intake (of a dry pellet containing: dry matter, 95%; crude protein, 44%; and
estimated digestible energy, 15 kJ/g) of acclimatised fish increased
essentially linearly with water temperature (over the range of 20 to 29°C) and
fish size (Figure 2A); expressed as a function of fish biomass, food intake
declined allometrically with fish size (Figure 2B).
Absolute
growth rate increased linearly with fish size at each water temperature (Figure
3).
Increasing
the feeding frequency from 1 to 3 times daily increased food intake, slightly
especially for smaller fish
Assessment of
nutritive value of feed ingredients
Measurement of the
apparent digestibility of a feedstuff is essential if diets are to be
formulated to meet prescribed nutrient specifications at least cost. Because of the difficulty if not
impossibility of collecting the total daily faecal output of an aquatic animal,
apparent digestibility is typically measured using indirect procedures
employing digestibility markers.
Differences in the concentrations of the marker and of the particular
nutrient in the food and in representative samples of faeces allows
digestibility to be derived from the equation: (<100 g), but the extra food
did not result in better growth rate. A similar observation had earlier been made
by Tucker et al. (1988). Analysis of the data generated the following
food intake prediction equation:
lnDFI
= -7.285 + 0.478lnW + 0.391T -0.0065T2 + 0.074F (R2 = 0.97)
where ln is the natural logarithm, DFI is daily food
intake (g/fish/d), W is weight (g) of the fish, T is water temperature (°C) and
F, the number of feeds/d.
ADNut
= 100 * [ 1 - {(MFI/MFO) * (NutFO/NutFI)}]
where AD is apparent
digestibility (%); M and Nut are the concentrations (% dry matter) of the marker
and nutrient respectively in the food (FI) and faeces (FO).
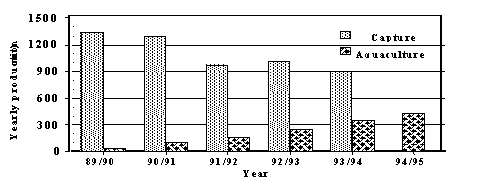
Figure 1. Production of barramundi
from capture fisheries and aquaculture in Australia
We have found Ytterbium acetate
(at 0.05 to 0.1% of diet) to be a more reliable digestibility marker than
chromic oxide. Its analysis however,
requires a mass spectrophotometer. Apparent digestibility measurements were
made using substitution procedures with the test ingredient being substituted
in a basal diet at amounts of not less than 30%. We found faecal samples
collected by sedimentation resulted in an over- and under-estimation of the
apparent digestibility of protein and lipid respectively, because of the rapid
leaching of soluble N which was almosthalf of the total N in the faeces
(Windell et al., 1978; Smith et al., 1980; Williams et al.,
1996). While intestinal dissection is the sacrificed and the procedure is very
labour intensive. However, stripping of
lightly anaesthetised fish has proved to be a reliable and efficient method for
collecting faecal samples. From the
the data on crude protein and energy apparent digestibility values for a number
of dry feed ingredients commonly available in Australia (Table 1), barramundi
are capable of digesting the protein from a wide variety of animal and plant
feedstuffs but that they are less well able to digest the energy contained in
terrestrial animal and plant food sources.
Protein requirement of juvenile
barramundi
Many different
approaches have been used with terrestrial and aquatic animals to define
essential amino acid requirements. The
most widely used (traditional) methodology involves feeding graded levels of
one amino acid at a time in a test diet containing either all crystalline amino
acids or a mixture of pure proteinsand crystalline amino acids. Disadvantages of this methodology are (i) it
is a slow process to evaluate each of the 10 or so essential amino acids; (ii)
absolute response to diets comprised mostly of crystalline amino acids is
usually inferior to that seen with diets based on intact proteins; and (iii)
the derived dietary amino acid level that maximises fish response will be
specific to the experimental conditions, particularly energy intake and the adequacy
of all of the other essential amino acids.
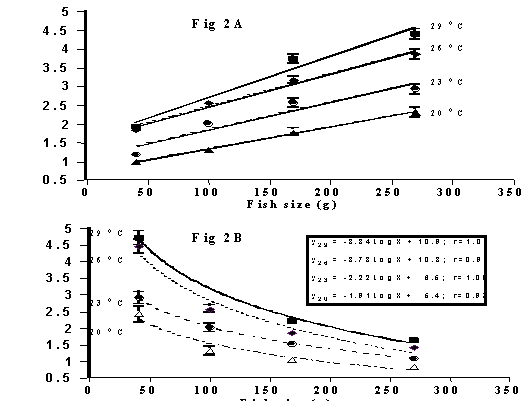
Figure 2. Effect of water temperature and fish size on intake of dry food pellet
by juvenile barramundi: A, daily food intake; B, percent of fish biomass
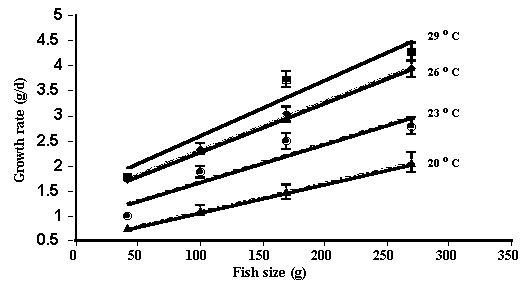
Figure 3. Effect of water temperature and
fish size on growth rate of barramundi
Table 1. The apparent
digestibility of air-dry feed ingredients for barramundi
|
Feed ingredient |
Apparent digestibility (%) |
Digestible energy |
|
|
|
Crude protein |
Gross energy |
(kJ/g) |
|
Fishmeal (Danish)1 |
88.7 |
99.2 |
20.0 |
|
Fishmeal (tuna)1 |
92.3 |
68.1 |
11.2 |
|
Meat meal (55% CP)2 |
75.1 |
76.3 |
13.4 |
|
Meat meal (50% CP)2 |
60.4 |
63.5 |
12.0 |
|
Poultry offal meal2 |
75.8 |
73.6 |
15.8 |
|
Soybean meal (full-fat)2 |
82.3 |
72.2 |
15.7 |
|
Soybean meal (solv)2 |
80.8 |
59.3 |
11.8 |
|
Canola meal2 |
80.0 |
54.2 |
10.7 |
|
sem (range) |
0.9-10.2 |
1.9-8.4 |
0.4-1.8 |
1 Determined by intestinal dissection (Williams et al., 1996).
2 Determined by stripping of fish (McMeniman et al., 1996)
An alternative methodology which has gained
considerable support over the last decade is the "ideal protein"
concept as espoused by Cole (1980).
'Ideal' protein is defined as one that is perfectly balanced in terms of
its amino acid content for the type of production required (viz for
growth, maintenance, reproduction). Such a protein would have the highest
possible biological value, i.e., the greatest efficiency of conversion
of dietary protein into deposited protein.
Once this is determined, dietary specifications can easily be tailored
for any given rate of growth (strictly speaking, for a given rate of protein
deposition) of the fish. If growth in
fish mirrors that seen in terrestrial monogastric animals such as pigs and
poultry, growth is expected to exhibit dependency and independency to both
protein and energy intake. The optimum
dietary protein to energy ratio can be determined by feeding increasing amounts
of protein (of constant quality) in conjunction with aconstant amount of
energy. The slope of the response line
(i.e., biological value) will
indicate how close the amino acid composition of this protein is to the ideal
pattern.
This approach was tested by formulating a
semi-purified diet in which all of the protein (of an amino acid composition
closely matching that of barramundi protein) was provided as a protein
mixture (reference protein). Protein
content of the diet was varied incrementally from 29 to 57% by adding the
reference protein at the expense of non-protein ingredients manipulated to
maintain the desired energy content (Table 2).
Fish were fed to satiety twice daily and held in water at 28°C for an
experimental period of 28 d. Production responses are tabulated in Table 3 and
Figure 4. As the inclusion content of the reference protein increased, there
was a marked curvilinear reduction in food intake and a corresponding although
less marked improvement in food conversion (P<0.05). These effects caused growth rate to exhibit
a bent stick response, increasing linearly up to a dietary protein content of
about 46%. This is similar to the recommendation of Boonyaratpalin (1989) that
the dietary crude protein content of grow-out Asian sea bass should be 45 to
50% (supplied predominantly from fishmeal).
When expressed as a function of absolute intake,
growth and food conversion improved curvilinearly (P<0.05) with increasing
protein intake with the response reaching an asymptote value at an intake of
1.44 g protein/fish/d (Figure 5A). In contrast, growth rate and food conversion
deteriorated with increasing digestible energy consumption (Figure 5B),
indicating that the response was clearly that of a simple protein
dependency. Based on this result, the
dietary protein to digestible energy dependency.
Table 2. Composition of the diets in the protein requirement experiment
|
Feed source |
Diet |
|||||
|
1 |
2 |
3 |
4 |
5 |
6 |
|
|
Formulation (%) |
||||||
|
Reference protein1 Starch (autoclaved) Diatomaceous earth Soybean oil Fish oil Vit + Min premix |
35.0 47.5 3.5 2.0 8.0 4.0 |
42.0 40.0 5.3 1.8 7.2 4.0 |
49.0 32.5 7.1 1.6 6.4 4.0 |
56.0 25.0 8.9 1.4 5.6 4.0 |
63.0 17.5 10.7 1.2 4.8 4.0 |
70.0 10.0 12.5 1.0 4.0 4.0 |
|
|
Chemical analysis |
|||||
|
Crude protein (%) Gross energy (kJ/g) Est dig. energy (kJ/g) Crude fat (%) |
29.0 18.93 15.0 11.7 |
34.6 18.85 15.0 11.0 |
40.1 18.78 15.0 10.2 |
45.7 18.70 15.0 9.5 |
51.2 18.63 15.0 8.7 |
56.8 18.55 15.0 8.0 |
1 Formulation (g/kg) of the reference protein was: Casein, 430; Fishmeal (Peruvian), 300; Gluten, 250; Lysine HCl, 5; d/l Methionine, 5.5; l Threonine, 2.5; l Tryptophan, 1; and NaHCO3, 6.
Table 3. Production
responses of barramundi to diets varying in protein content
|
Attribute |
Treatment (diet CP%) |
±sem |
|||||
|
|
29.0 |
34.6 |
40.1 |
45.7 |
51.2 |
56.8 |
|
|
Start weight(g) End weight (g) |
74.9 123.6C |
75.2 144.2B |
78.6 153.5A |
76.3 157.5A |
75.8 152.1A |
76.2 158.5A |
1.47 2.14 |
|
Food
intake (g/d) |
4.16A |
3.62B |
3.32C |
3.00D |
2.61E |
2.66E |
0.058 |
|
Growth
(g/d) |
2.38C |
2.51BC |
2.72AB |
2.90A |
2.72AB |
2.94A |
0.072 |
|
FCR
(g:g) |
1.76E |
1.44D |
1.22C |
1.04B |
0.96AB |
0.91A |
0.026 |
A,B,C,.D - Means without a common superscript letter differ (P<0.05).
Table
4. Effect of water temperature (WT) and essential fatty acid content (EFA) of
the diet on the growth performance of juvenile barramundi
|
WT |
EFA( EPA + DHA) content (mg/g) |
Response1 |
±sem |
|||||
|
(°C) |
5.1 |
8.3 |
11.5 |
14.6 |
17.8 |
21.0 |
(WT x EFA) |
|
|
|
Food intake (g/d) |
|
|
|||||
|
20 |
1.29 |
1.31 |
1.34 |
1.19 |
1.19 |
1.25 |
ns |
|
|
29 |
4.41 |
4.21 |
3.89 |
3.58 |
3.69 |
3.93 |
L; Q |
0.091 |
|
|
Growth rate (g/d) |
|
|
|||||
|
20 |
0.70 |
0.72 |
0.81 |
0.70 |
0.79 |
0.75 |
ns |
|
|
29 |
2.93 |
3.07 |
3.16 |
3.13 |
3.44 |
3.24 |
L; Q |
0.103 |
|
|
Food conversion (g:g) |
|
|
|||||
|
20 |
1.83 |
1.83 |
1.66 |
1.70 |
1.52 |
1.68 |
Q |
|
|
29 |
1.50 |
1.37 |
1.23 |
1.14 |
1.07 |
1.18 |
Q |
0.077 |
1 Response to diet at each water temperature: ns, not significant (P>0.05); L, Linear (P<0.05); Q, Quadratic (P<0.05).
At low water
temperature, fish response was unaffected by dietary fatty acid content,
whereas at high water temperature, food intake declined curvilinearly with
increasing n-3 fatty acid content (Figure 6).
This is because of a concomitant improvement in food conversion, growth
rate improved linearly up to a total EPA and DHA content of 17.8 mg/g (Figure
7).
The observed curvilinear response
of food intake to dietary fatty acid content at high water temperature was an
unexpected result. The increased food intake on the diets containing the lowest
n-3 fatty acid content could be interpreted as an attempt by the fish to
increase its intake of critical n-3 fatty acids. This is plausible since food conversion also showed a marked
deterioration for diets containing the lowest n-3 fatty acid content. The lack of response to dietary fatty acid
content by fish held at low water temperature was probably due to the reduced
growth and thus a minimal requirement for n-3 fatty acids. These results
indicate that the optimal dietary n-3 to n-6 fatty acid content should be not
less than 1.6:1 (equivalent to an EPA + DHA content of 17.8 mg/g) for rapidly
growing fish at high water temperature whereas at low water temperature the
ratio need not be greater than 0.6:1 (EPA + DHA content of 5 mg/g). In reviewing the essential fatty acid
requirements of marine fishes, Tucker (1992) concluded that a dietary EPA + DHA
concentration of 20 mg/g was a reasonable specification for the young of most
species but this could be reduced to 14 mg/g for older fish. Tucker (1992) stressed the essentiality of
DHA and advocated that it comprise at least half of the n-3 fatty acid content
of the diet. Boonyaratpalin (1989)
recommended that the total n-3 fatty acid content of the diet for juvenile
Asian sea bass should be 10 to 15 mg/g.
![]()
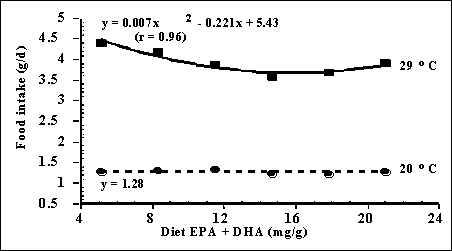
Figure 6.
Effect of dietary fatty acid content and water temperature on food intake of
juvenile barramundi
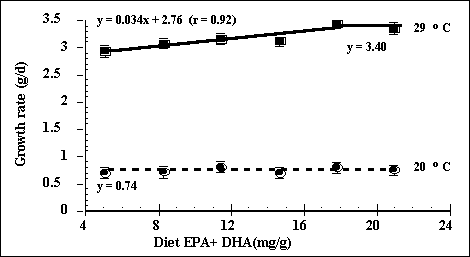
Figure 7. Effect of fatty acid content of the diet and water temperature on growth rate of Juvenile barramundi
Commercial
trailing of nil-fishmeal grow-out diets for barramundi
The
primary objective of the research program was to develop improved and cheaper
barramundi grow-out diets with a reduced dependency on fishmeal. Information on
the nutritive value of alternative feedstuffs and the fish's requirements for
key nutrients was used to formulate practical diets for commercial evaluation. Several laboratory and on-farm trials have
been done to demonstrate the suitability of these new generation diets. The results of a study comparing diets
formulated without any fishmeal with either a proprietary barramundi diet or a
high fishmeal experimental control diet are discussed to illustrate the
progress that has been made.
A 4x4 randomised block design was used to compare
three experimental diets (two containing no fishmeal) with a proprietary barramundi
diet, all being commercially extruded as dry floating pellets (Table 5). The ingredient cost of the nil-fishmeal
diets was 15 to 20% cheaper than that of the proprietary diet.
Cages (2m2) were stocked with 300 fish
(initially 226 ±16.3 g) and suspended in an aerated freshwater pond. Fish were fed once daily to satiety and
reared on the diets for 10 weeks. At
the conclusion of the feeding period, all fish were weighed and samples taken
to assess eating quality using taste-panel procedures.
There were significant (P<0.05) differences in fish
growth performance between the diets (Table 6). Food intake of fish on both of the nil-fishmeal diets (diets 2
and 3) was higher than on each of the other diets, indicating high
acceptability by the fish for the nil-fishmeal diets. Food conversion and growth rate on the high energy nil-fishmeal
diet (diet 2) were as good if not better (P<0.05) than all of the other
diets. Food conversion was best on the
fishmeal control diet (diet 1) but not significantly better (P>0.05)than
that for the high energy nil-fishmeal diet).
These results demonstrate that appropriately
formulated and cheaper diets without fishmeal (but containing some fish oil as
a source of n-3 fattyacids) are able to grow barramundi as well as those fed on
conventional high fishmeal diets.
Equally important, the eating quality of the fish reared on nil-
conventional high fishmeal diets.
Equally important, the eating quality of the fish reared on nil-fishmeal
diets was indistinguishable from fish fed on high fishmeal diets.
Assessment of the eating quality of the fish using
trained taste panels at the Queensland Government's Centre for Food Technology
showed similar scores for all diets (Table 7).
Scores for undesirable off-colours and flavours were very low and the
overall acceptance of the fish on all diets was very high. than that for the
high.
Further studies are continuing to specify requirements
of grow-out barramundi for critical essential amino acids and the role of high
energy diets for the commercial production of the fish.
The financial support of the
Australian Fisheries Research and Development Corporation and the Australian
Meat Research Council is gratefully acknowledged. We thank the Workshop
Organising Committee and in particular Dr. Michael Phillips of NACA and Mr.
Rooney Biusing of the Sabah Department of Fisheries for the invitation to
present this work.
Table 5. Composition of the diets fed in the on-farm trial
|
Attribute |
Diet
description and formulation |
|
||||
|
|
Diet
1 (Control) |
Diet
2 |
Diet
3 |
Diet
4(Proprietary1) |
||
|
|
Formulation
(g/kg) |
|
||||
|
Wheat |
304 |
105 |
161 |
|
|
|
|
Chile
fishmeal (65% CP) |
350 |
0 |
0 |
|
|
|
|
Meat
meal (52% CP) |
0 |
500 |
500 |
|
|
|
|
Meat
meal (60% CP) |
100 |
0 |
0 |
|
|
|
|
Blood
meal (ring) |
0 |
90 |
70 |
|
|
|
|
Soybean
(fullfat) |
160 |
100 |
150 |
|
|
|
|
Gluten
(90% CP) |
50 |
100 |
50 |
|
|
|
|
l-lysine
HCl |
0 |
7.5 |
6.5 |
|
|
|
|
d/l
Methionine |
1.5 |
3 |
3 |
|
|
|
|
Fish
oil (Chile) |
25 |
60 |
50 |
|
|
|
|
Tallow |
0 |
25 |
0 |
|
|
|
|
Vit
& min mixture |
9.5 |
9.5 |
9.5 |
|
|
|
|
|
Chemical analysis |
|
||||
|
Gross
energy (kJ/g) |
19.2 |
21.0 |
19.6 |
20.0 |
|
|
|
Est
DE (kJ/g) |
15.0 |
16.4 |
15.3 |
nd |
|
|
|
CP
(g/kg) |
436 |
470 |
440 |
543 |
|
|
|
Fat
(g/kg) |
87 |
138 |
116 |
69 |
|
|
|
Arginine
(g/kg) |
27.4 |
29.4 |
28.8 |
29.7 |
|
|
|
Lysine
(g/kg) |
28.7 |
30.2 |
28.5 |
46.1 |
|
|
|
Meth
+ Cyst (g/kg) |
9.2 |
8.1 |
7.3 |
10.8 |
|
|
|
Threonine
(g/kg) |
17.4 |
16.0 |
15.2 |
23.9 |
|
|
|
C20:5
n-3 (g/kg) |
4.3 |
6.7 |
5.5 |
5.0 |
|
|
|
C22:6
n-3 (g/kg) |
7.5 |
8.9 |
7.4 |
9.1 |
|
|
1 Formulation of the proprietary diet is
confidential. nd, not determined.
Table 6. Effect of diet on performance
of barramundi reared under commercial farm conditions
|
Response
attribute |
Diets |
±sem |
|||
|
|
Diet
1 |
Diet
2 |
Diet
3 |
Diet
4 |
|
|
Food
supply (kg/wk/cage) |
7.6C |
9.1A |
9.1A |
8.3B |
0.10 |
|
Growth
rate (kg/wk/cage) |
6.2B |
7.0A |
6.4AB |
6.1B |
0.18 |
|
Farm
food conversion |
1.22A |
1.31AB |
1.44B |
1.37B |
0.041 |
A,B
within
response attributes, means without a common letter differ (P<0.05)
Table 7. Effect of diet on eating quality scores (0 = low; 100 = high) of fish reared under commercial farm conditions
|
Response
attribute1 |
Diets |
|||
|
|
Diet
1 |
Diet
2 |
Diet
3 |
Diet
4 |
|
Colour Yellow Grey |
6.9 10.5 |
8.8 10.5 |
9.1 9.7 |
7.6 9.5 |
|
Flavour Sweet Fishy Muddy |
19.2 49.0 14.8 |
22.4 47.3 13.9 |
21.7 45.5 15.9 |
18.1 46.8 16.6 |
|
Texture Firm Moist |
46.5 44.2 |
46.9 43.9 |
44.3 42.7 |
47.3 47.4 |
|
Overall
acceptability |
60.0 |
64.3 |
61.2 |
63.5 |
1 Differences between diets for all attributes were not
significant (P>0.05).
References
Barlow, C., K. Williams, L. Rodgers, C. Agcopra, I.
Hocking. 1996. Effect of water temperature and dietary w-3 to
w-6 fatty acid ratio on growth of juvenile Asian sea bass, Lates calcarifer
(Bloch). pp. 29-30. In: Proc. World Aqua. Soc. 29
January-2 February 1996, Bangkok.
Boonyaratpalin, M.
1989. Seabass culture and
nutrition. pp. 43-77. In: Akiyama, D. M. (ed.) 'Proceedings
of the People's Republic of China Aquaculture and Feed Workshop'. Singapore: American Soybean Association.
Braaten, B. R. 1978. Bioenergetics - A
review on methodology. pp. 462-501. In:
Proc. World. Symp. Finfish Nutr. Fishfeed Tech. Hamburg, 20-23 June 1978.
Cole, D. J. A. 1980.
The amino acid requirements of pigs - the concept of an ideal
protein. Pig News Info. 1:201-205.
McMeniman, N. 1996.
In: Allen, G.L. (ed.)
FRDC Progress Report - Fishmeal Replacement in Aquaculture Feeds for
Barramundi, April 1996. Canberra, Australia: Fisheries Research &
Development Corporation.
Smith, R.R., M.C. Peterson, and A.C. Allred. 1980.
Effect of leaching on the apparent digestion coefficients of feedstuffs
for salmonids. Prog. Fish Cult. 42: 145.
Steffens, W. 1989.
Energy requirement. pp. 184-208. In: Principles of Fish
Nutrition. Chichester, UK: Ellis
Horwood Ltd.
Talbot, C.
1993. Some aspects of the
biology of feeding and growth in fish. Proc. Nutr. Soc. 52: 403-416.
Tucker Jr. , J. W.
1992. Marine fish
nutrition. pp. 25-40. In:
Allen, G. L. and W. Dall
(eds.). Aquaculture Nutrition Workshop.
New South Wales Fisheries, Salamander Bay, Australia.
Tucker Jr., J. W., M. R. MacKinnon, D. J. Russell, J.
J. O'Brien, and E. Cazzola. 1988.
Growth of juvenile barramundi (Lates calcarifer) on dry feeds. Prog.
Fish Cult. 50: 81-85.
Williams, K., C. Barlow, J. Rose, B. Kelly. 1996.
Effect of faecal collection method on the apparent digestibility of
diets for Asian sea bass, Lates calcarifer (Bloch). pp. 438-439. In: Proc. World Aqua. Soc. 29
January-2 February 1996, Bangkok.
Windell, J.R., J. W.
Foltz, and J. A. Saroken. 1978. Methods of faecal collection and nutrient
leaching in digestibility studies. Prog.
Fish Cult. 40: 51-55.