Status of Grouper Breeding and Culture in Thailand
R. Yashiro[1]
Abstract
Epinephelus malabaricus, E. coioides, E.
tauvina, E. salmonoides, E. lanceolatus, E. fuscoguttatus, Lutjanus
argentimaculatus, L. johnii, Plectropomus maculatus, and Cromileptes altivelis
are being cultured for local and overseas consumption using seed supply mainly
from the wild. Small traps, push net,
shelter trap, and gill netting are used for catching fish juveniles. Only Lates calcarifer seeds are
supplied by government and private hatcheries.
Hatcheries produce occasionally Epinephelus malabaricus, E.
coiodes, E. tauvina, E. salmonoides, and Lutjanus
argentimaculatus due to improper management of broodstock and nursing. The breeding and grow-out of groupers (Epinephelus
spp.) in Thailand were attempted but there are constraints. The cage and
earthen pond culture systems for these groupers including aspects of culture
such as stocking density, feed, feeding of grouper, are reviewed and discussed.
The fish (mainly alive) are exported to Malaysia, Singapore, Taiwan, and Hong
Kong at 5.6 to 21.6 $US/fish (0.5 to 1.3 kg), depending on the season. Ninety percent of the fish farmers culture
grouper in cage which set in natural sea-water along the coastal areas at the
southern and eastern part of Thailand. Recently about 10-20% of shrimp ponds
are being converted to fish culture and may continue to increase. Environmental pollution and diseases are
causes of loss of profit in fish culture and farming. Diseases of culturing marine fish are discussed separately.
Introduction
More than 40 species of
groupers were reported (Sirimontrapom, 1994) in Thai waters. Many of these species (Epinephelus spp.,
Plectropomus spp., and Cromileptes sp.) are cultured in Thailand
for some time now. They are suitable culture
species because they are easy to grow and fast-growing. Furthermore, the
groupers have desirable white, tender, tasty meat that Asians find desirable.
These species are cultured in cages along the coastal areas in the eastern and
southern Thailand. This is in response
to the high demand for these fishes in domestic and international markets.
The cage culture of groupers
followed the decline of the shrimp-farming industry. Since 1990, many shrimp farms are forced to stop their operations
because of diseases and environmental problems. The shrimp farmers began to culture brackishwater fish (seabass)
and marine fishes (groupers) in the former shrimp ponds. The main supply of grouper seeds is from the
wild.
From this beginning, grouper
culture is progressing in Thailand. The
techniques, status, and constraints of breeding, nursing and grow-out
techniques of grouper, Epinephelus coioides and E. malabaricus,
that are presently produced and cultured in Thailand, are reported in this
paper. Aside from these groupers, other
marine fishes are also cultured in Thailand.
The production of major species, based on trends over the past 5 years,
major culture system types (Figure 2) and geographical location of culture systems
are reported in this paper (Table 3).
Cultured Species
Eight
species of groupers are cultured along the coast of Thailand. The Malabar grouper, Epinephelus
malabaricus, and the orange-spotted grouper, E. coioides, are
the two major species that are being cultured because of its economic importance. The six minor species also being cultured
are the greasy grouper, E. tauvina, the giant grouper, E. lanceolatus,
the areolate grouper, E. areolatus, the brown-marbled grouper, E.
fuscoguttatus, the spotted coral grouper, Plectropomus maculatus,
and humpback grouper, Cromileptes altivelis. Aside from these groupers, the seabass, Lates calcarifer,
and the red snapper, Lutjanus argentimaculatus are major species of
marine fishes being cultured in Thailand.
These fishes are primarily inhabitants of shallow coastal reefs,
although some species range into deeper water of above 100 m.
Overview
Culturing of grouper
Groupers
are sluggish fish always found rest quietly among the rocks or hiding among the
corals. They like to live in waters
with salinity ranging from 12 to 30 ppt and temperature range from 22°C to
28°C. The fish farmers culture groupers
both in cages and earthen ponds but most (about 90%) of farmers culture them in
cages that are set in seawater. The
cage culture of grouper is being conducted at provinces along the coastal areas
at the southern and eastern part of Thailand, i.e., Suratthani,
Chumporn, Nakhonsri-thamarat, Songkhla, Pattani, Satul, Krabi, Trang,
Phang-nga, Phuket, Chachengsao, Rayong, Chantaburi, and Trard. The pond culturing
are found in some provinces as Pachaburi, Suratthani, Nakhonsri-thamarat,
Songkhla, Satul, Chantaburi and Trard.
The majority of grouper seeds for culturing were obtained from the wild,
because seeds from hatchery were limited, due to unstable seed production and
nursing techniques (Chen et al., 1977; Ruangpanit et al.,
1988;Sakares and Sukbantaung, 1987).
Marketing and trading of live fish
Grouper
seed from the hatchery go directly to the fish-farmers that will then be
cultured either in cages or in ponds.
On the other hand, natural seeds go through the middleman before these
reach the farmer to culture and grow.
When the fish has attained marketable sizes, they are supplied direct to
fine domestic consumers or export to Hong Kong, Singapore, Taiwan, and
Japan. The flow charts of trading and
prices of grouper and other marine fishes produced from natural seeds and from
hatchery-produced seeds are in Appendix 1 and 2.
Grouper breeding and larval rearing
Breeding
The
research on breeding of groupers has begun more than 20 years ago. The first success in mass propagation of
grouper seeds was achieved in 1988 at Tamano association in Japan. The sea-farming association produced over
100,000 fry of Epinephelus akaara (Fugunaka et al. 1990). In 1989, the same grouper was reported to
produce as high as 403,000 fry (Murayama et al., 1993). In Thailand, attempts breeding of other
species of groupers, such as E. tauvina, E. salmonoides, E. malabaricus,
and E. suillus, were not as successful (Julavitayanukul et al.,
1985; Sakaras and Kumpang, 1986; Rattanachot and Ningnoi, 1986; Hunsopa et
al., 1990), Kungvankij et al., 1986; Ruangpanit et al., 1988;
Doi et al., 1991). Aside from
breeding, induction of spawning and seed production by environmental or water
management method and hormonal injection were attempted by these researchers.
The fry production from these species is still under 100,000. Eventually, mass
propagation of grouper seeds at National Institute of Coastal Aquaculture was
attained during 1991-1993 until present but production is still inconsistent.
Broodstock preparation and
breeding
Grouper
broodstock were cultured in net-cage 5x5x2.5m at Nu Island where the water
salinity is around 28-34 ppt throughout the year. They were transferred to the 150-tonne, round-shaped, concrete
ponds during September each year. The physico-chemical characteristics of the
concrete ponds are maintained at 30-31 ppt and 28-30°C and by changing 50-80% of the water
daily. The broodstock was fed with
fresh sardines every day at 1-2% of their body weight. Vitamins and minerals were added into the
diet by embedding capsules of these into the sardine before feeding. In particular, Vitamin E (4,000 mg/kg),
Vitamin C, fish oil, and Premix 0.3 g/kg are added for better growth
performance.
Table 1.
Number of grouper brood
stock used in breeding 1991-1993
|
Year |
Male |
Weight (kg) |
Female |
Weight(kg) |
Remark |
|
1991 |
15 |
11-15 |
15 |
5-6.5 |
30 Fish/tank |
|
1992 |
20 |
10-15 |
30 |
3.5-7.5 |
|
|
1993 |
9 |
9.5-12 |
21 |
4.5-7.5 |
|
Source: Ruangpanit et al., 1993a
The
grouper-breeding season in the wild is during October to March each year and
the females lay eggs at night from 2100-2400 H. To induce breeding and spawning in the hatchery in 1991 to 1993,
male broodstock weighing 9.5-15.0 kg and female 3.5-7.5 kg were chosen and were
stocked in the spawning tank at the ratio of 1:1 or 1:2 male:female (Table 1). The groupers were subjected to two methods,
namely, by water manipulation and by hormone injection. Details of the procedure are summarised as
follows:
·
water manipulation: 80% of water were changed 5 days before full moon or new moon. New water was added continuously to allow
water overflow until about 80 % of water have been replaced. Grouper broodstock
stimulated by water manipulation laid eggs 1-2 days before full moon or new
moon. The females laid eggs 2-3 years
for an average of 4-10 days each time.
A better hatching rate was obtained by this method. The broodstock can be injected later on with
hormones for further rematuration and spawning. (As this does not use hormones,
it is considered in this paper a “natural spawning” (Table 2)).
·
hormone injection: Puberogen, the commercially
available hormone, was used at dosages of 100 IU/kg and 50 IU/kg body weight of
female and male broodstock, respectively.
The broodstock are first checked for suitability of this procedure. A sample of the female oocyte was examined.
If the oocyte size is over 400 micrometers, it is considered suitable for
inducing maturation by this hormone.
The male should have white milt flowing when the abdominal part is
gently pressed. The hormone injection
should be given 2-5 days before the new moon. The broodstock release their eggs
3-9 days after hormone injection for 5-16 days continuously.
The results of these two methods of breeding
induction and spawning are different.
Egg production is lower by water manipulation than with hormones but
hatching rates, survival rates, and spawning frequency are slightly higher
(Table 2). The number of spawning days
was from 10-23 days by water manipulation while, for hormonal induction, ranged
from 7-33 days.
Table 2.
Number of eggs, hatching rates of grouper, and other aspects of spawning
under two conditions during 1991-1996 (NS, natural spawning; HI, hormonal
injection)
Year
|
Aspects
|
|||||||||
|
|
Eggs (x106) |
Larvae (x106) |
Hatching rate (%) |
Spawning (occurrence) |
Spawning Duration (in days) |
|||||
|
|
NS |
HI |
NS |
HI |
NS |
HI |
NS |
HI |
NS |
HI |
|
1991 |
4.16 |
11.97 |
1.81 |
4.76 |
43.51 |
39.76 |
3 |
1 |
23 |
10 |
|
1992 |
|
32.37 |
|
11.16 |
|
34.47 |
|
3 |
|
33 |
|
1993 |
6.75 |
7.02 |
3.05 |
2.2 |
45.19 |
31.33 |
2 |
1 |
10 |
9 |
|
1994 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
1995 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
1996 |
- |
4.23 |
- |
1.37 |
- |
32.47 |
- |
1 |
- |
7 |
|
Total |
10.91 |
55.59 |
4.86 |
19.47 |
|
|
5 |
6 |
33 |
59 |
|
|
|
|
|
|
44.55 |
35.06 |
|
|
|
|
Hatching
Grouper
eggs are 800 to 900 microns and are of the floating type. The floating eggs are collected, the
following morning after induced-spawning procedures, washed, and placed into
the funnel-shaped fibre glass tank with a capacity of 500 li. The water used for hatching has salinity of
30 to 3l ppt and pre-treated with chlorine or calcium hydrochloride at 20
ppm. The eggs are kept in the tank with
the density of 500-1,000/li. The
fertilised eggs hatch within 17-19 hours at 26 to 29°C and the larvae were then
transferred to the nursing pond.
Larval Rearing
Preparation of nursing pond and water management
Concrete
tanks with the volume of 25 tonnes and dimension of 4x6x1.5m were used for
larval rearing. The pre-treated water,
used for hatching was re-used to fill half of the tank. Aeration was used and water temperature was
kept at 27-30°C. During nursing, green
water (Chlorella sp.), about 1 ton was poured into the tank everyday,
until the tank filled up.
Water
is changed throughout the nursing period.
Ten to 20% of water was changed every day. At Day 20, Day 30, and Day 45 of nursing, the daily rate of
water change was increased to 30%, 40% and 50%, respectively. The bottom cleaning was done everyday during
Day 12 to Day 19 by siphoning out sediments and dirt and every 3-5 days after
Day 20 or when fry start to school at the bottom of the tank. Later when the fry can start feeding on
fresh feed and mixed pellets, the cleaning of the bottom of the tank was done
every day.
Larval feeding
The
mouth of larval groupers opens around 53 to 59 hrs after hatching and 6 hours
later the fry begin feeding (Ruangpanit et al., 1993b). Thus, a variety
of feeds are necessary. After Day 2 or
the 48th hour, the fry are then fed daily with small rotifers (Brachionus
plicatilis), that can pass through 125-micron mesh nets, at 10-15
individuals/ml, and the sea Chlorella at 1 ton. All sizes of rotifers
will be added from the Day 6 to Day 29.
During this time, fry were trained to feed on Artemia from Day l5
and supplemented with copepods and Moina. Adult Artemia was given at about Day 25. Artemia
nauplii and adult should be enriched by emulsified fish liver oil at 50 ppm
19-22 hr before feeding or by commercially-enriched essential fatty acid
(n3-HUFA 450mg/g) 200-300 ppt for 12-15 hr or 6-10 hr (Figure 1). Emulsified fish oil was prepared by using 50
ml of fish liver oil, 2 raw chicken eggs yolk, and 200-300 ml of water mixed in
the blender for 2-3 minutes. This mixture
was added to 1 tonne of seawater, composing of 200 li of green water (Chlorella
sp.) and 800 li stocked with 100 Artemia/ml.
|
Age ( day) |
5 |
15 |
20 |
30 |
40 |
45 |
50 |
55 |
|
|
1991-1992 |
|
|
|
|
|
|
|
|
|
|
Rotifer |
|
|
|
|
|
|
|
|
|
|
Artemia nauplii |
|
|
|
|
|
|
|
|
|
|
Copepod, Moina |
|
|
|
|
|
|
|
|
|
|
Adult Artemia |
|
|
|
|
|
|
|
|
|
|
Fish meat |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1993 |
|
|
|
|
|
|
|
|
|
|
Rotifer |
|
|
|
|
|
|
|
|
|
|
Artemia nauplii |
|
|
|
|
|
|
|
|
|
|
Adult Artemia |
|
|
|
|
|
|
|
|
|
|
Fish meat |
|
|
|
|
|
|
|
|
|
|
Pellet feed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Source: Ruangpanit et al.,
1993
Figure 1. Feeding scheme for grouper fry-rearing
Nursing management
Cannibalism
in groupers is high at Day 35 to Day 40.
Shelters must be put for increase survival rates of fingerlings. PVC
pipes with a diameter of 2" were cut into 30-50 cm and were grouped by 4-6
pieces to hang along the edges and corners in the tank. By removing the fingerlings found in the
shelter pipes 2-3 times daily could do the “sizing” of fingerlings.
Sources and types of seed/fingerlings
1-2
cm seeds/fingerlings: Almost all grouper seeds are
obtained from the wild because seed production from the hatchery is still
insufficient and the supply is unstable.
To catch fingerlings, fishermen place aggregating devices or traps made
of dried twigs along channels in the mangrove areas and other brackish waters
along the coast. Every 2-3 hours or
longer, these traps are lifted up and fingerlings are collected with scoop nets
and then transported to the middle man or broker who sells it to the fish-farmers.
Another procedure used by some fishermen is the gill net with a small mesh size
(0.5-1.0 cm). The gill net block is
placed across the known path of passing grouper fingerlings. The fingerlings are then collected by
lifting the net and scooped up. The
former method always cause mass mortality of fingerings when nursing due to the
stress during trapping and transportation. This method is becoming more popular
because it can catch more fingerlings compared to the gill-netting method. Push nets are also used to capture the
fingerlings.
2.5-10 cm juveniles: Juveniles of this size are collected mainly with small fish traps
locally called ‘sai’ of ‘lob’ (20x40x20cm) or with round traps
with diameter of 20 cm and length 20-25 cm.
Juveniles of giant grouper are caught by bigger traps (40x60x40 cm) or
even bigger in some area as Pang-nga and Phuket. The frame of these traps are
made of bamboo or mangrove branches and fixed with nylon net or plastic screen
mesh size of 1-2 cm. The traps are set
every evening at the brackish water or salt-water canal in mangrove areas or
hidden in the reef area. Fishermen
retrieve their traps the following morning or a few days after the deployment
of the traps, depending on their sense of water currents and lunar cycles. Gill nets are also used for catching fish
juveniles of this size range in some areas.
Grouper Culture Systems
Fingerling to juvenile
Grouper fingerling size of
1.0 to 2.5 cm from wild and from fish hatchery mostly are nursed in concrete
tank, nylon net cage and earthen pond up to 8.0 to 12.0 cm, then transferred to
fish cages or earthen pond for grow-out to marketable size.
Fingerling nursing in
concrete tank.
Nursing of groupers can be done in 1 m3 round tank or 5 to 30-t round
or rectangular-shaped concrete tanks.
They were stocked with fingerlings of 4.3 cm at 15 to 50 individuals/m2
and nursed up to 9 to 10 cm for 60 days.
They were fed with fresh fish and wet or moist and dry pellets
(Panbanpeaw and Sakaras, 1990) to satiation once or twice per day. In
high-density stocking rates, concrete blocks are used as shelters. Grading or sizing is needed for homogenous
growth and for minimising loss due to cannibalism.
Fingerling nursing in nylon
net cage:
Nursing fingerling grouper in net-cages (1x1x1.5 m3) is better than
in concrete ponds because stocking density is higher and growth rate is better
(Chulawitayanukul et al., 1988).
Stock fingerlings of 5.7-cm (3.1 g) at 300 to 500 individuals/m2
fed with trash fish for 75 days can produce juveniles of 14.1 cm (49.9 gm) with
91.1 % survival in salinity 15.2 ppt.
Fingerling nursing in earthen pond: Earthen ponds measuring
800 m2 up to 3,200 m2 are always used for nursing grouper
fingerling. These ponds are stocked at
25 to 100 individuals/m2 and fed with trash fish. At about 45 to 60 days, individuals attain
the size of 10 to 15 cm. These
juveniles are partially or totally harvested by seine and sold or transferred
to be cultured in net-cages and earthen ponds. Survival rates are rather low
compared to the other two methods above but fishermen do not want to spend time
in sizing individuals for culture.
Cage culture
Location of
grouper cage culture
Majority of fish farmers
likes to culture groupers and other marine fishes in cages because of
convenience and lower cost of investment compared to culture in ponds. Cage culture is versatile as it can be
operated in brackishwater area near mouth of the river, along the coast, or on
islands in open sea. The cage culture
system also eliminates the problems of water management that are faced in
pond-culture. The cage culture of
seabass were demonstrated by the Department of Fisheries under the Bay of
Banjul Project (BOB) in the southern part of Thailand and became very popular
because of the above advantages. Most
of fish-cages are located in the coastal provinces in southern Thailand, i.
e., Trung, Pang-nga, Krabi, Satul, Nakornsri-tummarat, Songkhla, Phuket,
and Ranong and some along the eastern coast of Thai Gulf, i. e. Chachoengsao,
Rayong Chartaburi, and Trard (Table 3).
Table 3. Location, total numbers of cages, and number of for grouper culture in Thailand
|
Province |
Area (rai) |
Area (ha) |
No. of cage |
Grouper cage |
|
Chumporn |
2.25 |
0.36 |
147 |
79 |
|
Suratthanee |
836.7 |
133.87 |
357 |
- |
|
Nakornsrithamarat |
356.7 |
57.07 |
130 |
102 |
|
Patalung |
129.01 |
20.64 |
520 |
- |
|
Ranong |
36.56 |
5.85 |
834 |
435 |
|
Phuket |
9.28 |
1.48 |
600 |
503 |
|
Phang-nga |
28.37 |
4.54 |
3,979 |
2,055 |
|
Krabi |
34.6 |
5.51 |
542 |
270 |
|
Trang |
17.18 |
2.75 |
559 |
475 |
|
Satul |
164.66 |
26.35 |
1,970 |
1,734 |
|
Pattani |
51.38 |
8.22 |
1,553 |
2 |
|
Naratiwat |
8.45 |
1.35 |
1,392 |
64 |
|
Songkhla |
371.67 |
59.47 |
1,220 |
<10 |
|
Chachuangsoa |
- |
- |
2,000 |
- |
|
Rayong |
- |
- |
10 |
10 |
|
Chantaburi |
- |
- |
50 |
|
|
Trard |
- |
- |
60 |
5 |
Design and construction of
fish-cage
There are 2 types of
fish-cages, namely, the fixed and floating cages. Both cages are either square or rectangular and comes in several
sizes, e. g., 1.5xl.5x2m3, 2x2x2.5m3, 3x3x2m3,
4x4x2m3, 5x5x2m3, and 10x10x3m3, etc. The type of cage used by fishermen depends
on the water depth and level of investment.
Fixed cage - This type of fish-cage has
the nets fixed to strong and stable posts, so the cage cannot move up and down
along with tidal movement. The areas
suitable for this cage type should be not deeper than 2.5 m and the tidal
difference between highest and lowest tidal levels should be about 50 to 60
cm. This type is popular in the eastern
provinces as Rayong Chantaburi, Trard and some in the southern provinces as
Chumporn Surattani, Songkhla, and Pattani.
A variation of this type is set at Aow Makarmpom, Klang district, and
Rayong province. The fish-cage has a
frame made of steel bars and fixed with nylon attached to it. It is set in deeper areas and is completely
submerged during high tide. Feeding of
fingerlings is done through the PVC pipe with a diameter of 10.0 cm.
Floating cage - This type is suitable for culturing in waters deeper than 2 m
and with tidal range of more than 1 m.
The net is tied within the raft or floater made of bamboo or styrofoam
and have steel bars to fix and reinforce the raft. This type is suitable for fish-cages that face to the open sea
such as in Satul, Trung, Krabi, Ranong, and Pang-nga. The floating net-cage can be constructed into 2 styles, with
frame and without frame. The cage with
frame is the one that is popular to fishermen.
Cage with frame The frame is either steel
bars with a diameter of 1 inch or wood of bigger diameter. The frame will help fix and reinforce the
net cage therefore allow good circulation of water through the cage.
Frameless cage - The top of the net is
tied to the float and the bottom is held down with sinkers at every
corner. Since this cage is frameless,
the shape of this type of net-cage changes according to the velocity of the
current. This type is suitable for
temporary stocking of fingerlings because the water circulation in the cage is
poor.
Besides the construction of
cages, several aspects are considered in the cage culture of groupers. The mesh sizes appropriate for nursery is 1
to 2 cm and for grow-out at 5 to 7 cm.
The materials for the raft and posts for fixing the cage should be
inexpensive, strong enough to stand wind and wave action, easy to clean, and
can be transported to another place.
The design of raft should convenient for culture and the net should have
no dead corners. The net-cage is fixed
to the floating raft with a distance of at least 30-cm from the water surface
level to allow circulation. The
distance of 1 to 3 m between cages and 3 to 5 m between rows of cage are
recommended also for good water circulation (Tipchid et al., 1984; DOF,
1986).
Stocking density and
production
The density of stocking has
been variable over the years. In
traditional cage culture for groupers, the stocking density 15 inds/m3
(DOF, 1980) but recently fingerlings were stocked at various densities from
30-125 inds/m2 (Table 4).
Table 4. Examples of stocking densities and yield/m2 of grouper in grow-out net cages
|
Fish size (cm) |
Fish size (gm) |
Stocking Density (inds./m2) |
Average Yield (kg/m2) |
References |
|
- |
61.2, 103.4, 149.4 |
30,45,60 |
16.8, 23.8, 29.7/ 6 months |
Sakaras & Sukbuntaung,
1985 (cage size1x1x1.5m3) |
|
10-15 |
20,30,60 |
60,75,90 |
27.8, 33.9, 37.9/ 6 months |
Sakaras & Kumpang,
1987a (cage size 1x11x1.5m3) |
|
15-30 |
80-300 |
12.5 |
7.5-9.4/6 months |
Priyiwatee, 1988 (cage size 4x4x2m3) |
|
16.2 |
83.7 |
75 |
31.8/6 months |
Sakaras & Kumpang, 1988
(cage size 2x3x1.5m3) |
|
- |
126.3 |
40, 50, 60 |
13.4, 15.5, 15.6/ 5 months |
Chulawitayanukul et al.,
1989 (cage size 1.5x3x1.5m3) |
|
12.3-16.9 |
628.6-67.1 |
75,100,125 |
31.6, 44.5, 53.7/ 6 months |
Sakaras et al., 1990 (cage size 1x1x1.5m3) |
Source: Ruangpanit and Yashiro
(1994)
The appropriate stocking
density of grouper cage culture in brackishwater (21-25 ppt, 29-30°C, pH 7.33, D.O.
5-6 mg/l with water flow 300-500 m3/hr), based of dissolved oxygen
budget (Nabhitaphata et al., 1988).
The lower optimum density was 58 inds/m3 or 75 inds/m2
with possible stocking limit of 351 inds/m3 or 457 inds/m2
for fish up to 500 gm, and 31 inds/m3 or 40 inds/m2 for
fish up to 1.2 kg with possible limit of 187 inds/m3 or 244 inds/m2. At high stocking densities of 100 and 125
inds/m2, with addition of artificial shelters, the production/cage
was higher but feed conversion rate (FCR) was lower (Sakaras et al., 1990; Table 4). However, there was no statistical difference on growth, survival
rate, and fitness of fishes cultured in optimum stocking density (75 inds/m2)
compared with those cultured at higher stocking densities (100 to 125 inds/m2).
As stocking density increases, yield
increases but the FCR decreases. Thus,
optimum-stocking density is 75 inds/m2 and the best stocking density
is at 90 inds/m2. At the
best stocking rate, production rate of 37.9 kg/m2 and food
conversion rate of 6.78 kg/fish was observed (Table 5).
Table
5. Grouper cage culture at various
stocking densities (6 months) which showed no difference on growth and survival
rate but production yields were higher with increasing stocking density
(Source: Ruangpanit and Yashiro, 1994)
|
|
Stocking density (inds/m2) |
||||||
|
|
30* |
45* |
60* |
75** |
90** |
100*** |
125*** |
|
Final
growth (gm) |
601.9 |
547.6 |
508.8 |
473.9 |
448.3 |
467.7 |
443.1 |
|
Survival
rate (%) |
93.3 |
97.0 |
96.7 |
95.5 |
93.7 |
95.7 |
96.8 |
|
Yield
(kg/m2) |
16.8 |
23.9 |
29.7 |
33.9 |
37.9 |
44.5 |
53.7 |
|
FCR:
kg fish |
8.82 |
- |
- |
6.33 |
6.78 |
5.2 |
4.8 |
*Sakaras and
Sukbuntaung, 1985, **Sakaras and Kumpang, 1987a, **Sakaras
et al.; 1990
This finding on the relationship
of stocking rate and feed conversion rate is very useful for an economically
viable cage culture operation given the culture site, soil substratum, and
water quality. To illustrate this, an
economic analysis of stocking rate and production was conducted for grouper (E.
malabaricus) cage culture at Tuppud district, Phang-nga province
(Priyawatee, 1988). A total of 420
cages were operated by 60 families (3 have >10 cages, 2 have 5 to 9 cages
and 55 families have 1 to 4 cages). The
financial investment involves expenses on fixed costs for infrastructure and
variable costs on operational costs.
The fixed costs for 4 cages was estimated 12,000 Baht (480 US$), with
depreciation cost at 4,000 Baht (160 US)/year (aging of equipment over 3 years)
for structures (cage construction, materials, raft, etc.). The variable cost is 41,000 Baht (1,640 US)
for operational cost (fish, feed and wage/day 6 months). Therefore, if the fixed cost of
investment/cage was excluded, the cost of operation will be 10,250 Baht
(US$450; may be plus 25-30% in 1994).
The farmers produced groupers weighing 400 to 800 g at 40 to 60 t/y at
an average selling price of 220[2] Baht (US$8.8/kg). This level of production and income could increase
if stocking density is increased 6 times more from 12.5 inds/m2 to the optimum stocking density
of 75 inds/m2.
Fingerling
to juvenile grouper
Cage culture management
Net-cage
culture is labour-intensive. Sometimes,
the net-cages are blocked by sediment since there are located in turbid waters,
near mouths of rivers. Molluscs and
seaweed sometimes block the mesh of net-cages that are located in the open
water. In addition, sediment and
attached mollusc and seaweed increased weight of the net-cages, resulting to
partial closure of the mesh. These blockage and partial closure of mesh may
cause diseases or parasite infestation on the cultured fishes because of bad
water circulation through and in the net.
Therefore, it is necessary to change nets used for culture to allow time
to clean and dry the netting material every 1 to 2 months, depending on the
rate of accumulation of unwanted dirt.
Raft, sinker, and float also have to be checked regularly. In high stocking density, sometimes partial
harvesting is recommended to eliminate the loss due to the cannibalistic habit
of groupers. Stocking fishes at
various times and rotation of harvesting is also practised to ensure continuous
income for small fish-farmers.
Water quality
Metabolic wastes from
cultured fishes are removed by water passing through the nets. Thus, the water quality in net-cages depends
on the current in the culture area. If
the current is weak, metabolic wastes accumulate at the bottom of the nets, and
the water quality inside the nets is poorer than that outside. If the tidal current is strong or heavy
fresh-water run-off during rainy seasons, there are no significant differences
in the water quality parameters among areas and between inside and outside the
cages (Table 6 and 7; Songsangjinda et al., 1993).
Table 6: Average water quality from grouper cage culture area of Khlong
Pakbara, Langu district, Satun province during the rainy season in October 6,
and 19, 1993. area 1: outside cage area (n=5), area 2: net cage under 3 years
old (n=3) and area 3: net cage over 3 years old (n=4). None of the differences were significant
(p>0.05)
|
|
Average water quality |
||||||||
|
Parameters |
Outside cage area |
net cage under 3 years old |
net cage over 3 years old |
||||||
|
|
range |
average |
SD |
range |
Average |
SD |
range |
average |
SD |
|
Dissolved Oxygen (mg/l) |
4.72-5.19 |
4.99 |
0.22 |
4.55-4.64 |
4.59 |
0.05 |
4.77-5.33 |
5.13 |
0.25 |
|
Oxidation
Reduction Potential (mv) |
183-215 |
200 |
12 |
177-188 |
182 |
6 |
171-219 |
198 |
22 |
|
Nitrite-nitrogen (mg/l) |
0.002-0.010 |
0.05 |
0.003 |
0.002-0.003 |
0.0027 |
0.0006 |
0.002-0.006 |
0.005 |
0.002 |
|
Nitrate-nitrogen
(mg/l) |
0.008-0.050 |
0.025 |
0.017 |
0.010-0.023 |
0.016 |
0.007 |
0.009-0.033 |
0.024 |
0.011 |
|
Total
ammonia -nitrogen (mg/l) |
0.07-0.077 |
0.036 |
0.026 |
0.004-0.026 |
0.018 |
0.012 |
0-0.45 |
0.019 |
0.022 |
|
Inorganic
nitrogen (mg/l) |
0.020-0.111 |
0.066 |
0.039 |
0.030-0.042 |
0.037 |
0.006 |
0.013-0.083 |
0.047 |
0.032 |
|
Organic
nitrogen (mg/l) |
0.453-1.039 |
0.75 |
0.284 |
0.314-0.836 |
0.598 |
0.264 |
0.360-0.922 |
0.609 |
0.285 |
|
Total
nitrogen (mg/l) |
0.531-1.071 |
0.818 |
0.255 |
0.535-0.866 |
0.635 |
0.260 |
0.389-0.985 |
0.656 |
0.281 |
|
Reactive
phosporus (mg/l) |
0-0.030 |
0.012 |
0.012 |
0-0.005 |
0.002 |
0.003 |
0-0.014 |
0.009 |
0.006 |
|
Total phosporus (mg/l) |
0.030-0.082 |
0.052 |
0.022 |
0.031-0.043 |
0.035 |
0.007 |
0-0.057 |
0.030 |
0.023 |
(Songsangjinda et
al., 1993)
Table
7: Average sediment quality from grouper cage culture area of Khlong Pakbara,
Langu district, Satun province during the rainy season in October 6, and 19,
1993. area 1: outside cage area (n=5), area 2: net cage under 3 years old (n=3)
and area 3: net cage over 3 years old (n=4).
All the average values are significantly different in each row
(p<0.05)
|
|
Average water quality |
||||||||
|
Parameters |
Outside cage area |
Net cage under 3 years old |
Net cage over 3 years old |
||||||
|
|
range |
average |
SD |
Range |
average |
SD |
Range |
average |
SD |
|
Nitrite-nitrogen |
0.074-0.096 |
0.082 |
0.008 |
0.057-0.314 |
0.149 |
0.143 |
0.086-0.778 |
0.288 |
0.330 |
|
Nitrate-nitrogen |
0.166-0.489 |
0.305 |
0.117 |
0.172-0.356 |
0.290 |
0.102 |
0.195-2.554 |
0.930 |
9.11 |
|
Total
ammonia-nitrogen |
2.03-3.16 |
2.64 |
0.48 |
2.11-5.81 |
3.80 |
1.87 |
3.12-36.33 |
12.62 |
15.87 |
|
Inorganic
nitrogen |
2.27-3.51 |
3.02 |
0.52 |
2.36-6.48 |
4.24 |
2.08 |
3.41-39.66 |
13.84 |
17.30 |
|
Organic
nitrogen |
66.92-217.4 |
133.36 |
60.65 |
147.89-254.19 |
206.20 |
53.9 |
426.9-1023.4 |
704.87 |
245.93 |
|
Total
nitrogen |
70.37-220.57 |
136.39 |
60.72 |
150.25-260.67 |
210.44 |
55.88 |
430.3-1030.9 |
718.72 |
245.58 |
|
Reactive
phosphorus |
0.196-0.409 |
0.343 |
0.087 |
0.87-1.74 |
1.24 |
0.45 |
2.46-12.99 |
5.19 |
5.20 |
|
Total
phosphorus |
6.75-21.0 |
13.93 |
6.62 |
7.37-80.62 |
32.96 |
41.32 |
62.75-78.12 |
72.66 |
7.05 |
|
Ignition
loss (%) |
0.59-2.10 |
0.78 |
0.21 |
0.54-3.37 |
1.83 |
1.43 |
4.13-16.21 |
8.04 |
5.65 |
|
Total
sulphide |
0-0.38 |
0.19 |
0.17 |
0-3.63 |
1.74 |
1.83 |
0.028-19.98 |
9.55 |
8.16 |
(Songsangjinda et al., 1993)
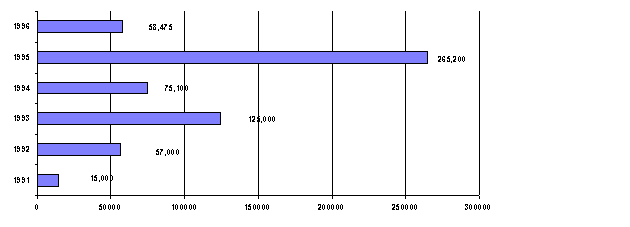 Figure 2. Production
of Grouper (Epinephelus malabaricus) juvenile age 50-55 days during
1991-1996
Figure 2. Production
of Grouper (Epinephelus malabaricus) juvenile age 50-55 days during
1991-1996
Pond Culture
Pond construction and layout
Seabass and grouper culture
ponds are constructed similarly as the coastal shrimp pond. The site should be carefully selected as
mentioned above. The size of seabass
rearing pond in the past was recommended to be at least 0.4 to 3.0 ha (4,160 to
31,200 m2) and 50 to 60 cm deep.
Smaller ponds are costly and inhibit the growth of fishes (Sirikul,
1982). Later, the Department of
Fisheries (1993) recommended that fish ponds should have area of about 800 to
1600 m2 and depth of 1.5 to 2.0 m deep because bigger ponds will be
under-utilised due insufficient number of juvenile fish fingerlings.
The fishpond
should have two water gates for the in-flow and out-flow of water. The pond
bottom should slope slightly toward the 30 to 40 cm deep passageway and deeper
than the pond to facilitate drainage.
Some farms use PVC pipe (16-20 cm in diameter) to pass through the dike
for this purpose. The dike should be
wide (3 to 6 m wide) for working on and compacted well to be strong.
Fishpond management
Pond preparation has to be
done before the culture of any fish.
The pond is flooded-out 2 to 3 times before cleaning by draining and
pumping out all water at low tide. The
mud and other dirt are removed, leaving the clear bottom exposed to sunlight
for 2 to 3 days to 1 week. Lime is
then used to disinfect and kill some pests that still left on the surface
(0.06-0.12kg/m2). The pond
is filled at high tide and then fertilised with chemical or organic fertilisers
for good natural feed (plankton) to bloom before juveniles are released to the
pond. In the case of big fingerlings
(200-300 g), fertilising the pond may be omitted. During culture, 40% to 70% of water change from the natural
source should be done everyday to maintain good water quality. A water reservoir is now necessary for
fishpond culture as well as shrimp culture.
At least 30% of total area should be a reservoir for stocking of water
before pumping to the culture ponds.
This practice can prevent infection by parasites and diseases.
Nutrition, Feed, and Feeding
Majority of grouper culture
operations in Thailand use trash fish or fresh fish as feed, i. e., the
yellow-striped trevally (Selaroides leptolepis), sardines or
fringe-scale sardinella (Sardinella fimbriata), and round scad (Decapterus
russelli), etc. These fishes
have high protein level and are suitable for cannibalistic species. The protein level of 50% was reported to
give maximum weight gain for grouper, Epinephelus tauvina (Teng et
al., 1977; Sukhawongs et al., 1978).
The feeds and feeding
schedule for some groupers are similar to seabasses (Chua and Teng, 1978;
Sakaras, 1990; Boonyaratpalin et al., 1993b; Wannagowat, 1994). Like seabass, E. tauvina fed once
every 2 days will greatly enhance maximum feed intake and efficient utilisation
of fish food. The formulated feed for
seabass and vitamin C were utilised successfully by E. malabaricus. Commercially extruded feed with slow sinking
rate is available in some Asian countries at present. This diet contains more than 73 % and 6 % crude protein and fat,
respectively, and it has less than 16 % ash, 3% fibre, and 12 % moisture
(Boonyaratpalin, 1993).
The feeding rate and food
conversion rate (FCV) was better with dry pellets than using fresh fish and wet
formulated feed in juvenile groupers (31.7-71.6 g; Panbankaew and Sakaras,
1990). The feeding rates were 2.99 %,
11.11%, and 8.81 % for dry pellet, wet formulated feed, and fresh fish,
respectively. The FCRs per kg were
1.22, 5.03, and 3.98. The FCR of trash
fish for 6 months cage culture of grouper were reported as high as 8.82:1 at
stocking densities of 30, 45, and 60 inds/m2 (Sakaras and
Sukbuntaung, 1985) and the lowest was 4.8:1 when stocking density was increased
to 125 inds/m2 (Sakaras et al., 1990).
Diseases and Parasites
Diseases
Diseases are one of the
major causes of nursery and grow-out mortality posing the principal constraint
in the future development of grouper and seabass cultures. About 10 years after the successful
production of seabass seeds in 1974, many diseases have been reported in
juveniles (> 200 g). Some fungal
diseases were reported in freshwater seabass culture but not in brackishwater
(Ruangpan, 1985). Various bacterial
diseases including Aeromonas spp., Flexibacter spp., Vibrio
spp., and Streptococcus spp. were identified from the sick fishes
(Danayadol, 1984; Ruangpan, 1985; Direkbusarakom and Danayadol, 1987). Bacterial infection was followed by viral
diseases in the lymph (lymphocystis) and kidney which is caused by unfresh
trash fishes (Limsawan et al., 1983; Danayadol et al.,
1984). Last year, 1993, a new viral
disease occurred in the broodstock (5-7 kg) of seabass in southern Thailand
(Danayadol et al., 1994). The
fish is pale (no blood), lethargic, anorexic, and bloated with excess abdominal
fluid. The sick fishes rest at the
water surface and die within 2-3 days.
The resulting mortality is about 60% of broodstock and this is a big loss
to a hatchery. The present knowledge and experience on treatment of viral diseases
of grouper and seabass are variable and fragmentary.
Parasites
Some parasites have reported
to be the main cause of mortality in juveniles and sometimes in adult
fishes. The ciliated protozoa are the
most important parasites that cause high mortality in seabass cage culture
(Ruangpan, 1985). These protozoa always
attack 10 to 20-day fry and 2 to 3-month juvenile seabass. There are 5 groups of protozoa that are
pathogenic:
1.
Icthyopthirious and 2) Cryptocaryon, occurring in freshwater and brackishwater,
respectively; these cause 'white spot' or 'ich' diseases - These protozoa will
attach to the gill filaments and disturb the normal functions of the
gills. The fish have to swim up to the
surface and gasp for air sometimes.
Some fish may scrape the net-cage or pond side and have wounds on the
skin. Fishes attacked by these
parasites will have small white spots on the skin first at the fins and along
the body surface. In some cases, have
fin rot or uncompleted scales, resulting to a weakened and anorexic fish. The
fish sometimes becomes vulnerable to attack by bacteria and die soon.
3. Trichodina - This protozoon attacks
the gills. The affected fish have pale
gills and are covered with mucus. This
protozoan always attacks fingerlings and juvenile fish.
Epistylis - This protozoon attacks seabass cultured in freshwater. With its stalk, this protozoon attaches
itself to the skin, gills, and eyes.
Oodinim - This flagellated protozoon causes the yellowish velvet skin
disease. When it attacks the gills, the
gill filaments fuse together, swell, and appear dark-reddish.
A variety of non-protozoa
also cause diseases and mortality to cultured fishes. The monogenic trematode worm always attacks the fingerlings and
juveniles but occasionally only the adult. The worms attach to the gills and
suck blood from host. Parasitic crustaceans sometimes cause more than 70 %
mortality in small fish cage culture.
The copepods Caligus sp. and Ergasilus sp. were reported
to have attacked seabass during the beginning of the rainy season in
Chantaburi, Rayong, Prjoubkinkhan, and Songkhla. The isopod, Aega sp., was also reported to be the cause of
huge losses for cage culture during rainy season (Ruangpanit, 1985).
Environmental
Considerations
Marine finfish culture could
result to eutrophication. Marine fish
farming create farm sediment, which consists of excess food and faeces, that
accumulate in the substrate of a farm. After years of cage culture operation,
the substratum beneath the cage gets worse due to the accumulation of organic
wastes. High level of organic waste in
sediment tends to increase a level of eutrophication and consumption of
dissolved oxygen in the culture area (Aure and Stigebrandt, 1990).
Constraints
Grouper breeding and hatchery
The constraints in breeding
and hatching groupers are outlined below:
1.
Quality and manipulation of broodstock - Further studies on the
quality of broodstock in terms of management and nutrition are necessary in
order to get good quality eggs and sperms at the right time.
2.
Hatching techniques - The batch hatching method gave the lower hatching
rate than the flow- through method but was more convenient (Ruangpanit et
al., 1993b) and therefore is still use in most fish hatcheries. A more
efficient hatching procedure has to be developed.
3.
Egg collection technique - Fertilised eggs of grouper which float type were
collected the next morning which are sometimes exposed to too much sun
light. This technique may cause low
hatching rate or alter the quality of the larvae.
4. Nutritional problems - Broodstock and fry nutrition still
have many point to be research in order to get good quality fry and high
survival rate. The mass mortality of the 20-day old fry was essential fatty
acid deficiency (Ruangpanit et al., 1993b; Pechmanee et al.,
1989; Maruyama et al. (1993). Dhert et al. (1991) reported that
they could reduce the mortality rate grouper fry by adding adequate amounts of
fatty acid into the feed.
5. Diseases problems - Various diseases, bacterial and viral,
are still encountered during nursing and grow-out periods. Knowledge of diseases and treatment are
needed to maintain good health of cultured fishes.
Grouper aquaculture
1.
Seeds - Inconsistent supply of
seeds from the hatchery to supply 3 government-owned grouper hatcheries and
private hatcheries are operated, and only one government hatchery at NICA can
mass-produce every year since 1990.
2.
Feeds –
Food supply is still dependent on fresh trash fish. A pellet feed for grouper should be developed.
3.
Environmental management of the culture and adjacent areas – The management of the
environment is still constraint as the drain-out of fish-farming add to the
impacts of shrimp-farming and agriculture in the same area
4. Culture techniques – The culture techniques for
both cages and ponds should be developed continuously in order to make culture
grouper in a sustainable manner.
Literature Cited
Aure, J. and
A. Stigebrandt, 1990. Quantitative estimates of the eutrophication effects of
fish farming on fiords. Aqua.
90:135-156.
Boonyaratpalin,
M. 1993. Nutrition requirement of grouper, Epinephelus. p. 50-55. In
Proceeding of Grouper Culture, Nov. 30-Dec, 1, 1993. Songkhla, Thailand.
Buranapanidgit,
J., M. Boonyaratpalin, T. Watanabe, T. Pechmanee, and R. Yashiro. 1988.
Essential fatty acid requirement of juvenile seabass, Lates calcarifer.
Tech. Paper No. 3/1988. National Institute of Coastal Aquaculture Kao Saen Soi
1 Muang District, Songkhla, Thailand. pp 21.
Chua, T. E.
and S. K. Teng. 1982. Effect of food ration on growth, condition factor, food
conversion efficiency, and net yield of estuary grouper, E. salmonoides
(Maxwell), cultured in floating net cages. Aqua. 27: 273-283.
Chungyampin
S., B. Sirikul, C. Chanchuglin, S. Techanarawong, and W. Watanakul. 1983.
Nursing seabass larvae in net cages from 1.5-%cm with different initial
stocking density. In Annual Report 1983. National Institute of Coastal
Aquaculture, Songkhla, Department of Fisheries. p. 54-71. (in Thai).
Chungyampin,
S., C. Chanchuglin, B. Sirikul, B., S.
Techanarawong, and W. Watarakul. 1983. Experiment on rearing 2-4 inches seabass
young fish, Lates calcarifer at different density. In Ann. Rep.
1983. National Institute of Coastal Aquaculture, Songkhla, Department of
Fisheries. pp. 72-80. (in Thai)
Danayadol, Y.
1984. Study on prevention prophylactic and treatment of diseases in
seabass. Ann. Rep., 1984. Songkhla
Fisheries Station, Department of Fisheries. pp.143-152. (in Thai)
Danayadol, Y.
and S. Direkbusarakom. 1987. The
diseases of Grouper (Epinephelus rnalabaricus, Bloch & Schneider)
Contribution No. 1/1987. National Institute of Coastal Aquaculture Kao Saen Soi
1 Muang District, Songkhla Thailand. 6 pp. (in Thai).
Danayadol, Y.
S. Direkbusarakom, and S. Boonyaratpalin. 1994. Epizootic haematopoietic
necrosis, a new viral disease in seabass spawner, Lates calcarifer,
cultured in Thailand. Tech. Paper No. 6/1994. National Institute of Coastal
Aquaculture, Kao Saen Soi 1, Muang District, Songkhla, Thailand. 12 pp. (in
Thai with English Abstract)
Department of
Fisheries. 1993. Manual for Brackishwater Fish Culture, Department of
Fisheries, Ministry of Agriculture and Cooperative. 38 pp. (in Thai).
Dhert, P., L.
C. Lim, P. Lavens, T. M. Chao, and R. Chou.
1991. Effect of dietary essential
fatty acids on eggs quality and larviculture success of the greasy grouper, Epinephelus
tauvina, F.): Preliminary Results.
Pp. 58-62. Larviculture
Symposium ’91. Lavens, P. P. Sorgeloos,
E. Jaspers, and E. Ollevier. Spec.
Publ. Euro. Aqua. Soc. No. 15.
Direkbusarakom,
S. and Y. Danayadol. 1987. Diseases of
seabass caused by non-hemolytic Streptococcus sp. Tech.
Paper No. 6/1987. National Institute of Coastal Aquaculture, Songkhla,
Department of Fisheries. 11 pp. (in Thai with English Abstract).
Doi, M.,
M. Nawi bin Hj. Mohd., N. R. bin Nik
Lah, and Z. bin Talib. 1991. Artificial propagation of the grouper, Epinephelus
suillus, at the marine finfish hatchery in Tanjong Demong Terengganu,
Malaysia. Dept. Fish., Min. Agr.,
Malaysia. 41.
Fukunaga et
al 1990
Hunsopa, Y.,
T. Jindamaikul, S. Sukaputh, W. Riewthong, and T. Duangsa. 1990. Study on
breeding and larval rearing of red spot grouper, Epinephelus tauvina,
Phuket Coastal Development Culture Center Tech. Paper No. 36, 24 pp.
Julavitayanukul,
P. 1987. Study on effect of vitamin E for the development of grouper, Epinephelus
tauvina, eggs. Review of the
research on grouper culture conference 23-25 February 1987 at NICA, pp.
122-129.
Julavitayanukul,
P., C. Putinowarat, and N. Suteemechaikul. 1987. Study on breeding of grouper, Epinephelus
tauvina. Review of the research on grouper culture conference 23-25
February 1987 at NICA pp. 74-81.
Kungvankij,
P., L. B. Tiro. B. Pudadera, and I. O. Potestas. 1986. Induced spawning and
larval rearing of grouper (Epinephelus salmonoides Maxwell), pp. 663-666. In J. L. Maclean, L B.
Dizon, and L.V. Hosillos (eds). The first Asian Fisheries Forum. Asian
Fisheries Society, Manila, Philippines.
Maruyama K., K.
Nogami, Y. Yoshida, and K. Fukunaga. 1993. Hatchery technology on the breeding
and seed production of red spotted grouper, Epinephelus akaara, in
Japan. Japan Sea-Farming Association, Goto station, Tarnanuora, Nagasalii pp.
02-05.
Nabhitabhata,
J., R. Prempiyawat, K. Klaokling, and S. Kabinrum. 1988. Estimation on optimum stocking density of grouper, Epinephelus
tauvina (Forskål), in cages on basis of dissolved oxygen budget: Pang-rat
river Rayong Brackishwater Fisheries Station Brackishwater Fisheries Division
Department of Fisheries. 27 pp. (in Thai with English Abstract).
Panbanpaew,
S., and W. Sakaras. 1990. Experiment on Sea Grouper, Epinephelus malabaricus,
with dry pellet. Tech. Paper No. 38/1990. Rayong Brackishwater Fisheries
Station, Brackishwater Fisheries Division Department of Fisheries. 17 pp.
Patarapinyo,
W., K. Silapajam, and J. Nugranad. 1987. Experiment on rearing of seabass, Lates
calcarifer (Bloch), in earthen pond. Tech. Paper No. 44/1987.
Prachuabkhirikhan Brackishwater Fisheries Station, Brackishwater Fisheries
Division, Department of Fisheries. 7 pp. (in Thai with English Abstract).
Pechmanee, T.,
M. Assavaaree, P. Bunliptanon, and P. Akkayanon. 1989. Possibility of
using rotifer, Brachionus plicatilis, as food for early stage of grouper
larvae, Epinephelus malabaricus.
Pp. 109-112. Report of the
Workshop Shrimp and Feed Development, Johore Bahru, Malaysia. 25-29, Oct. 1988. ASEAN/ST/89/GEN/11.
Ruangpan, L.
1985. Diseases and parasites found in seabass cage culture. Tech. Paper No.
1/85, January 1985. Coastal Aquaculture Research Sub-division, Brackishwater
Fisheries Division, Department of Fisheries. 16 pp. (in Thai).
Ruangpanit,
N. 1985. Final Report Besut Integrated
Fisheries Development Project Malaysia, Tanjong Demong Hatchery-Besut,
Malaysia. Consultant, FI: GCP/MAL/009/ CAN., pp11-17.
Ruangpanit, N.
1993. Technical manual for seed production of grouper, Epinephelus
malabaricus. National Institute of Coastal Aquaculture, Songkhla,
Department of Fisheries, Thailand. 46 pp.
Ruangpanit,
N., P. Bunliptanon, T. Pechmanee, P. Arkayanont, and J. Vanakovat. 1988.
Propagation of grouper, Epinephelus malabaricus, at National Institute
of Coastal Aquaculture, Songkhla, Tech. Paper No. 5/1988, National Institute of
Coastal Aquaculture, Songkhla, Departrnent of Fisheries, Thailand. 16 pp. (in
Thai with English Abstract).
Ruangpanit,
N., P. Bunliptanon, and J. Kongkumnerd.
1993a. Progress in the propagation and larval rearing of the grouper, Epinephelus
malabaricus. p. 32-42 In: The Proceeding of Grouper culture, Nov. 30
- Dec. 1, 1993. National Institute of Coastal Aquaculture, Songkha, Thailand
and Japan International Cooperation Agency, 133 pp.
Ruangpanit,
N., R. Yashiro, P. Bunliptanon, and V.
Vattanakul. 1993b. Hatching and biology
of early stages of grouper, Epinephelus malabaricus. p.45-49. In:
The Proceeding of Grouper culture, Nov. 30 - Dec. 1, 1993. National Institute
of Coastal Aquaculture, Songkhla, Thailand and Japan International Cooperation
Agency, 133 pp.
Ruangpanit, N.
and R. Yashiro. 1994. A review of grouper and seabass culture in Thailand. 35
pp. In: The proceeding of seabass and grouper culture, Hawaii 1994.
Sakaras, W.
1990. Experiment on seabass, Lates calcarifer (Bloch), cultured in cages
with dry pellet. Tech. Paper No. 5/1990. Rayong Brackishwater Fisheries
Station, Coastal Aquaculture Division, Department of Fisheries. 15 pp. (in Thai with English Abstract).
Sakaras, W.
and S. Sukbuntang. 1985. Experiment of
grouper, Epinephelus tauvina (Forskål) in cage with different stocking
Density. Technical Paper No.
1/1985. Rayong Brackishwater Fisheries
Station, Brackishwater Fisheries Division, Department of Fisheries. 28 pp. (in Thai with English Abstract).
Sakaras, W.
and P. Kumpang. 1987a. Effect of
stocking density on growth and production of estuary grouper, Epinephelus
tauvina (Forskål), cultured in Cages. Tech. Paper No. 3/1987. Rayong Brackishwater Fisheries Station,
Brackishwater Fisheries Division, Department of Fisheries. 24 pp (in Thai with English Abstract).
Sakaras, W.
and P. Kumpang. 1987b. Comparative
study on growth and production of Seabass, Lates calcarifer (Bloch),
Cultured in cages with freshwater and mixed diet. Tech. Paper No. 14/1987.
Rayong Brackishwater Fisheries Station, Brackishwater Fisheries
Division, Department of Fisheries. 14
pp (in Thai with English Abstract).
Sakaras, W.
and P. Kumpang. 1988. Growth and production of brown spotted
grouper, Epinephelus tauvina (Forskål) cultured in cages. Rayong Brackishwater Fisheries Station,
Brackishwater Fisheries Division, Department of Fisheries. 17 pp (in Thai).
Sakaras, W.,
S. Sangpradap, and Y. Soodmee. 1990.
Experiment on increasing production of brown spotted grouper, Epinephelus
tauvina (Forskål), by using artificial hides to increase stocking
density. Rayong Brackishwater Fisheries
Station, Brackishwater Fisheries Division, Department of Fisheries. 24 pp (in Thai with English Abstract)
Sirikul, B.
1982. Stocking and rearing of seabass
in grow-out ponds and cages. Lecture
notes. 15 pp.
Songsangjinda,
P., P. Na-anan, and D. Tanvilai. 1993.
Water and sediment quality in grouper cage culture area in Klong
Pakabara, Lag District, and Satul Province. pp. 112-119. In: The Proceedings in Grouper
Culture, Nov. 30 -Dec. 1, 1993. National
Institute of Coastal Aquaculture, Songkhla, Thailand and Japan International
Cooperation Agency, 133 pp.
Sukhawongs,
S., N. Tanakumchep, and S. Chungyampin, 1978.
Feeding experiment on artificial diet for greasy grouper, Epinephelus
tauvina, in nylon cages. Ann. Rep.,
Songkhla Fisheries Station, Depart.
Fisheries. pp. 103-117 (in
Thai).
Thanomkiat, T.
1987. Culture ofGrouper in floating net
cage with artificial diets. Tech. Paper
No. 26/1987, Phuket Brackishwater Fisheries Station. 17 pp. (in Thai).
Appendix 1. Flowchart of grouper production and
trading from natural seeds and from hatchery-produced seeds
Natural seeds
Natural Seeds (by shelter trap, gill or push netting) -------->Nursing (pond or cage)------>(0.04-0.1$US/1.5cm, 0.08-0.24$US/2-3 cm through middleman, 1/2-1/3 less from fisherman)
Culture (pond or cage)-----> Marketable size------> sold to
consumer or middleman for exporting -------> keep for selling to be
broodstock
Remark: The pricelist is in Appendix 2 for marketable sizes.
Hatchery-produced seeds
Eggs ------------------->
Seeds from Hatchery ------------> Nursing (pond or cage) ------->
(350-400/million of egg)
Culture (pond or cage)-----> Marketable size------> sold to
consumer or middleman for exporting -------> keep for selling to be
broodstock
Appendix 2. List of prices of groupers, snappers, and
other marine fish (25 B =1$US; nd = no data available)
|
Species |
1.2-1.5 kg/pc Live/kg ($US) |
0.5-1.0kg/pc Live/kg ($US) |
0.8-1.2kg/pc Dead/kg ($US) |
|
Epinephelus malabaricus |
10-16.8 |
6.0-10.0 |
3.4-4.8 |
|
E. coioides |
7.2-8.4 |
5.6-8.4 |
4.2-4.8 |
|
E. tauvina |
7.2-8.4 |
4.5-8.4 |
nd |
|
E. lanceolatus |
28.0-36.0 |
12.0-24.0 |
2.0-4.0 |
|
E. salmonoides |
7.2-8.4 |
Nd |
nd |
|
E. fuscoguttatus |
12.0-16.0 |
Nd |
nd |
|
Lutjanus argentimaculatus |
4.4-4.8 |
Nd |
3.0-3.6 |
|
L. johnii |
4.4-4.8 |
Nd |
nd |
|
Plectropomus maculatus |
18.0-22.0 |
Nd |
nd |
|
Cromileptes altivelis |
60.0-68.0 |
12.0-20.0 |
nd |
|
|
|
|
|