The
Status of Seed Production of Grouper and other Coral Reef Fishes in the
Philippines
Gerald F. Quinitio[1]
Abstract
Initial work on seed production of grouper in the Philippines was first
reported in 1986. Most of the work has
been undertaken by the SEAFDEC Aquaculture Department (SEAFDEC/AQD), which
concentrated on Epinephelus coioides.
The achievements and results of SEAFDEC/AQD on breeding and larval
rearing of grouper which including studies on sex inversion will be
discussed. Directions for future
research directions are also presented.
Introduction
Many
coral reef fishes are very important commercial fish species. Some species like groupers and snappers are
highly esteemed food fish while others are collected and used as aquarium
display. Their habitat, the coral
reefs, has been destroyed through destructive fishing. The culture of groupers and snappers is
becoming extensive especially in Southeast Asia due to increasing demand. However, expansion is hindered by unreliable
and limited supply of seeds for stocking in grow-out ponds and cages. At present, most of the seeds are collected
from the wild and is slowly becoming scarce. Several research institutions have
been doing work on grouper and snapper seed production in an attempt to augment
the natural seed supply.
In the
Philippines, trials to spawn and rear the larvae of groupers have been
undertaken by research institutions as well as in the private sector (Quinitio
and Toledo, 1991). The Aquaculture
Department of the Southeast Asian Fisheries Development Center (SEAFDEC/AQD)
has been doing most of the research work.
Garcia (in press) has reported the achievements until 1992 on the
breeding of groupers and snappers while Quinitio and Duray (in press) have
reported on larval rearing. Research on
marine ornamental fishes is a very recent activity due to demand in the fish
aquarium industry. Success in seed
production of marine ornamental fishes will also alleviate the fishing pressure
of these fishes and help rehabilitate coral reefs. In this review, I will discuss mostly the research work that have
been undertaken by SEAFDEC/AQD until the present since there is very limited
work on breeding of grouper, snapper, and marine ornamental fishes in other
research institutions and the private sector.
Grouper
Spawning
Kunvankij et al. (1986) conducted initial work
on grouper breeding and larval rearing. al. (1986). They showed that Epinephelus
malabaricus (=E. salmonoides) could be naturally spawned in
captivity after hormonal injection.
Females (3.6-6.5 kg) with mean egg diameter of 400 µm and males (10-16
kg) with running milt were injected with human chorionic gonadotropin (HCG) +
Chinese carp pituitary gland (CPG) and luteinizing hormone-releasing hormone
analogue (LHRHa). The induced pair of
fish was placed in separate cages installed in another tank. The fishes given 500 IU HCG + 3 mg of CPG/kg
body weight (BW) for the first injection followed by 1,000 IU HCG + 3 mg CPG/kg
BW after 24 h spawned naturally 12 h post-injection. At lower dosages of 500 IU + 3 mg CPG at 12-h or 24-h interval
and 10 µg LH-RHa/kg fish, eggs can be artificially fertilised by stripping.
The first natural spawning of captive grouper E.
coioides (=E. suillus) in the Philippines occurred in SEAFDEC/AQD on
4 July 1990 in fishes reared in a 50-ton concrete tank (Table 1; Toledo et
al., 1993). Six mature females
(3.5-5.0 kg) and 4 mature males (7-12 kg) were stocked in the spawning
tank. Spawning was observed from July
1990 to June 1991 except in May. Each
spawning run lasted for 5-17 times with the onset occurring within a 3-day
period before or after the last quarter moon.
Natural spawning of this grouper species was also observed in floating
net cages (4x4x3 m) that was stocked with a mature female and two spermiating
males. However, spawning was observed
only from July to October 1990 with each spawning run occurring for 5-10
times. The onset of spawning is similar
to that in tanks.
Table 1.
Natural spawning of Epinephelus coioides (= E. suillus) in
tank (50 tons) and floating net cage (4x4x3 m; from Toledo et al., 1993)
|
Holding System |
No. of Fish |
Spawning Period |
spawning run1 |
|
|
|
|
|
No. of Times |
Total Eggs (x106) |
|
Tank |
4M : 6F2 |
Jul 1990-Jun 1991 (except
May) |
5-17 |
0.5-15.8 |
|
Net Cage |
2M : 1 F |
Jul-Oct 1990 |
5-10 |
2.3-3.9 |
|
1 Onset of spawning is over
a period of 3 days before or after the last quarter moon 2 Two
males and one female died in November and one male died in April F = female; M = male |
||||
The groupers E. summana, E. caeruleopunctatus,
E. macrospilus, and E. fuscoguttatus have also been reported to
spawn naturally in captivity (Alava, in press). However, fertilised spawns were obtained only in E.
fuscoguttatus (100%), and E. summana (71%). Quinitio and Toledo (1991) reported that the
private sector have also spawned naturally E. malabaricus by injecting
100 µg LH-RHa/fish twice at 24-h interval.
Sex
Inversion
Most if not all species of grouper that have been
closely studied are protogynous hermaphrodites (Shapiro, 1987), i.e.,
they are female during the early stage of their life cycle and become male
during the later phase. This feature has posed a disadvantage since there are
no available males during the first maturation of a batch of fishes being
developed for broodstock. The use of
hormones to change the sex of cultured fish has been applied in several
fishes. In groupers, several studies
have been undertaken to inverse the sex of females in an attempt to have
functional males for spawning.
The androgen 17alpha-methyltestosterone (MT) is the
widely used hormone that is administered by oral, by injection, or by
implantation method. Results of work done at SEAFDEC/AQD are shown in Table
2. Tan-Fermin et al. (1994)
showed that two-year old juvenile E. coioides (mean BW: 1.2 kg) can be
sex- inversed to male when given 0.5-5.0 mg MT/kg BW injection every two weeks
for 5 to 6 months. However, the
sex-inversed juvenile groupers start reverting back to female after 4 months
and eggs can be cannulated after 8 months (Tan-Fermin, 1992).
Figure 1. Feeding and water management scheme
during larval rearing of grouper Epinephelus coioides (from Duray et al., in press)
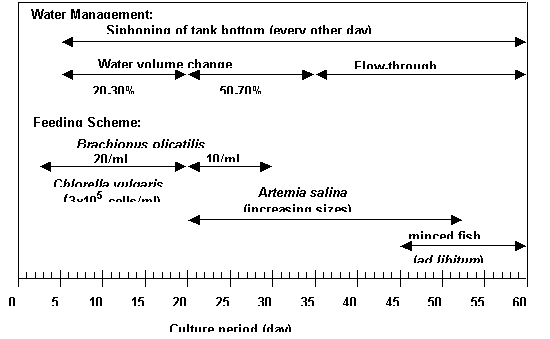
Table 2.
Successful sex inversion treatments on Epinephelus coioides at
SEAFDEC Aquaculture Department.
|
Treatment/Dose per kg BW |
Mode of |
Fish size/Stage |
Time to sex-inversion (months) |
Remarks |
References |
|
0.5-5.0
mg MT; every 15 days |
Injection |
1.2 kg |
5-6 |
Revert
to female when treatment was stopped. |
Tan-Fermin
(1992); Tan-Fermin et al. (1994) |
|
1.0
mg MT; every 2 weeks |
Injection |
2.2-5.1 kg mature |
7-10 |
Revert
to female when treatment was stopped. |
Marte
et al. (unpub.
data) |
|
4.0
mg MT; every 2 mon. |
Implantation |
-do- |
from 7 |
Observed
natural spawning. |
-do- |
|
4.0
mg MT + 20 µg LHRHa; every 2 mon. |
Implantation |
-do- |
from 6 |
|
-do- |
|
Social
control (one fish bigger than the other) |
- |
³ 5.0 mature |
from 2 (tank) from 4 (cage) |
No
inversion in some cases; Observed
natural spawning. |
Quinitio
et al. (unpub. data) |
|
MT
- 17a-methyltestosterone LHRHa
- Luteinizing hormone-releasing hormone analogue. |
|||||
In another work that was conducted in concrete tanks,
Marte et al. (1995) compared groupers (BW: 1.9-3.3 kg) which were given
implants (4 mg MT/kg BW) in May, August, and December (Group 1) and another
group given a single implant in December (Group 2). All fishes in both groups became functional males within 7-10
weeks after treatment and spawning was observed 5-6 times between February and
March of the following year. Although
higher fertilisation rate of spawns was observed in Group 1 (16-44%) than Group
2 spawns (3-12%), there were no hatched larvae from Group 1 whereas hatched
larvae from two spawns were obtained from Group 2.
Bigger-sized female E. coioides may also be sex
inversed by social control (Quinitio, unpub. data). When two females, one bigger than the other, were isolated from
the original group of floating net cage-reared grouper, all the bigger fish
(initial BW: 5.0-6.1 kg) changed to males.
The smaller ones (initial BW: 4.5-5.2 kg) remained females. This work was also tested in concrete tanks
stocked with 4 fishes. The biggest fish
(initial BW: 6.4 kg) was milting after 2 months while the smaller ones were remained
females. The fishes started to spawn on the fourth month. However, there may be cases wherein sex
change will not occur as observed in a tank stocked with two females. The bigger fish did not clearly shown signs
of sex inversion even after 16 months.
The above results clearly show that mature females of E.
coioides, when sex-inversed using hormone or social control, could become
functional males. However, it is not
known whether sex-inversed juvenile groupers could also be functional since no
pairing were conducted by Tan-Fermin et al. (1994).
Broodstock
Diet
The nutrition of the broodstock is an important factor
for gonad development and fecundity to insure good quality spawn (Watanabe,
1985). Groupers are carnivorous so the
feed given to the broodstock is fish by-catch (trash fish). Moreover, feed quality and quantity affect
spawning and egg quality. Toledo et.
al. (1993) mentioned that nutritional deficiency could be one of the
reasons for having inconsistent quality of spawns of E. coioides.
Therefore, an experiment was conducted in an attempt
to improve egg quality by enriching the different species of fish by-catch with
commercial HUFA (SELCO) or cod liver oil (Quinitio et al., 1996). Monthly egg production, spawning frequency,
fertilisation rate, egg viability, and hatching rate of fish fed by by-catch
alone were significantly higher compared to the grouper fed with SELCO-enriched
fish by-catch. These results suggest that varying the species of fish by-catch
could provide the requirements of E. coioides broodstock so as to
provide quality eggs. However, further work still needs to be done to improve
the quality of spawns and develop a proper artificial diet
Larval Rearing
The initial attempt to rear the larvae of grouper was
done in 1984 by Kunvankij et al. (1986) on E. malabaricus. A survival of 9% was attained on Day 20 when
larvae were first fed Isochrysis combined with sea urchin eggs until Day
13 and thereafter rotifer was given from Day 10.
Hatchery rearing of E. coioides had been first
attempted in 1990. Nagai (1990)
attained little success when larvae were reared at 10 and 20 larvae/l using
oyster trocophores as food during the first week followed by a microparticulate
diet (Nosan R-1) in combination with rotifers.
Realising the difficulty in rearing the larvae
of E. coioides, baseline studies that may be used in developing a
hatchery technique were then conducted.
It was observed that the growth and survival of grouper
larvae in small tanks (µ200 l) are optimum at 20 individuals/l stocking density
(Duray et al., 1995). Higher
survival could be attained in larger tanks (500 l) at a higher stocking density
of 30/l.
Studies on feeding biology showed that E. coioides
larvae start feeding on rotifers at Day 2 when they are about 2.6 mm total
length (TL) at an average of 1.3 individuals/larva (Duray, 1994). The amount ingested increased as larvae
grew. When Artemia was added on
Day 21, strong preference of this food over rotifers was observed. The larvae show a diurnal feeding pattern
with feeding incidence decreasing in the evening and becoming zero at 2100-2200
H. Active feeding starts earlier in
older larvae and satiation is between 0900-1100 H. Although larvae can be reared both in tan and black coloured
tanks during the first 14 days, they fed on more rotifers, grew better in
tan-coloured tanks using green water system of rearing (Duray et al., in
press, b). Larval survival was affected
by rotifer density and was highest at 20/ml.
A study on the effect of HUFA and vitamin C-enriched
rotifers on larval growth and survival of E. coioides reared for 24
days. Preliminary results showed no
significant difference in the survival of grouper larvae given Chlorella-fed
rotifers (Ch-R, 4.21%), rotifer-fed Culture Selco (CS-R, 2.56%), and Vitamin
C-enriched rotifer-fed Culture Selco (CSC-R, 1.89%). The last food-item was enriched with 20 % Vitamin C for 24
hours. However, larvae fed Ch-R had
poorer growth (8.39 mm TL; 3.26 mg wet weight, WW) compared to CS-R (9.74 mm
TL; 7.23 mg WW) and CSC-R (8.39 mm TL; 7.29 mg WW).
Duray et al. (in press c) described a protocol
for rearing E. coioides in the hatchery with a feeding regime composed
of Chlorella, rotifer, Artemia, and minced fish. The feeding and water management scheme is
shown in Figure 1. Higher survival
(19.8%) was observed in bigger tanks (3 tons) at Day 24 than in 0.5-tontanks at
Day 21 (7.4%). Feeding of screened
rotifer (<90 µm) during initial two weeks improved growth and survival of
the larvae. Older larvae seem to grow better at lower salinity of 24 ppt than
in 35 ppt.
Grouper
larvae seem to be poor feeders at the onset of feeding. To have small-natural food available, Doi et
al. (1996) used nauplii of coastal calanoid copepods Pseudodiaptomus
annandalei and Acartia tsuensis as well as rotifer as food from Day
3 to Day 10. Larvae fed copepod nauplii
had better survival and growth thereafter compared to those fed with rotifer
only. A 100% feeding incidence occurred
on Day 4 when copepod nauplii were given and only on Day 9 when rotifer alone
were fed. It seems that the grouper
larvae could catch the copepod nauplii easier than rotifer. In E. fuscoguttatus and E.
summana, Alava et al. (in press) achieved limited success in rearing
in 400-li or 3-ton tanks and feeding rotifer and Artemia. At Day 28 all the larvae died. Duray et al. (in press, a) developed
a hatchery rearing scheme for the red snapper based on several rearing trials
(Figure 2). It was best to rear the
larvae in bigger tanks (3 tons) and feeding them with screened rotifer (<90
µm) during the first 14 days. The
larvae fed with Artemia at 2/ml had higher survival (63.3%) when fed 4
times a day.
Marine Ornamental Fishes
Several
species of coral reef associated marine ornamental fishes are also being worked
out at SEAFDEC/AQD. At present, the
species being reared as broodstock include blue tang (Paracanthurus hepatus),
bannerfish (Heniochus acuminatus), damsel fish (Dascyllus aruanus),
and lemon damsel (Pomacentrus moluccensis) (G. Garcia, pers. comm.).
These were either collected from the coral reef areas or bought from
commercial traders in Manila. Natural
spawning was observed in several damselfish a month after stocking in an
aquarium. Six spawning events occurred
in one month. Attempt to rear the
larvae giving Pseudodiaptomus nauplii and small-sized rotifer as food
was not successful. Mass mortality
occurred at Day 4-5.
60
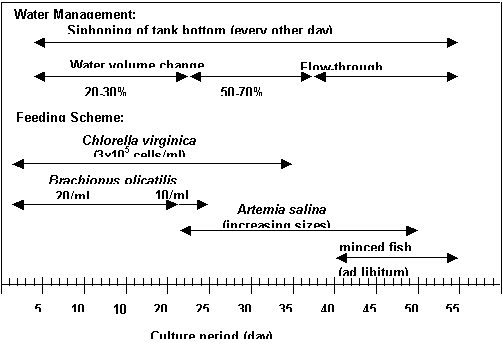
Figure 2. Feeding and water
management scheme during larval rearing of the mangrove red snapper Lutijanus
argentimaculatus (from Duray et al., in press )
Future Research Directions
To date, although spawning of E. coioides and L.
argentimaculatus could be achieved in the Philippines, there is still high
dependence on fish by-catch as feed to the broodstock. There is a need to develop a diet for
grouper and snapper broodstock so that fish by-catch could be eliminated or
reduced. The diet that will be
developed should also be able to provide the necessary nutrients that would
ensure good quality spawns. Spawning of
marine ornamental fish has only been achieved in only one of the reared
species. Further work has to be
undertaken to achieve this objective, especially on understanding the
reproductive biology of these fishes.
It seems that larval rearing of coral reef associated fishes is quite difficult. Research should be focused on determining the appropriate food especially during the early larval stages. The use of coastal calanoid copepods seem to give some promising results on grouper but it did not give good results in the damsel fish. Another area that should be looked into is larval physiology. Such basic information will serve as guide to developing a reliable hatchery technique. The use of thyroid hormones in fishes to improve larval survival is very promising. Research on this aspect should be continued in grouper and should also be tested on snapper and the marine ornamental fishes.
Literature Cited
Alava, M. N. R., M. L. L. Dolar, and J.
A. Luchavez. (in press). Natural spawning of four Epinephelus species
reared in the laboratory. In: C.
L. Marte, G. Quinitio and A. Emata (eds.).
Proceedings of the Seminar-Workshop on Breeding and Seed Production of
Cultured Fishes in the Philippines. 4-5
May 1993, Tigbauan, Iloilo, Philippines.
SEAFDEC/AQD, Tigbauan, Iloilo, Philippines.
Chavez, D. R., D. G. Estenor, G.
Merchie, and P. Lavens. 1995. The effect of HUFA and vitamin C-enriched
rotifers on larval growth and survival of grouper (Epinephelus suillus). p.
170. In P. Lavens, E. Jaspers and I. Roelants (eds.). Larvi'95 - Fish and Shellfish Larviculture Symposium. European Aquaculture Society Spl. Publ.
24. (Abstract)
de Jesus, E. G. 1996.
Do thyroid hormones play a role in the metamorphosis of the grouper, Epinephelus
coioides p. 259-260.
In J. Joss (ed.). The
Third Congress of the Asia and Oceania Society for Comparative
Endocrinology. 22-26 January 1996,
Macquarie University, Sydney, Australia.
Doi, M., Toledo, J. D. Golez, de los S.
N., M. Santos, and A. Ohno. 1996.
Feeding performance of early red-spotted grouper, Epinephelus
coioides, larvae reared with mixed zooplankton. A paper presented at the International Symposium on Live Food
Organisms and Environmental Control for Larviculture of Marine Animals. 1-4 September 1996, Nagasaki, Japan.
Duray, M. N. 1994. Daily rates of ingestion of rotifers and Artemia
nauplii by laboratory-reared grouper larvae, Epinephelus suillus. Philipp.
Scientist 31: 32-41.
Duray, M. N., L. G. Alpasan, and C. B.
Estudillo. (in press, a). Improved
hatchery rearing of mangrove snapper, Lutjanus agentimaculatus in larger
tanks and with smaller rotifer Brachionus plicatilis and more Artemia. Israeli J.
Aquaculture (Bamidgeh).
Duray, M. N., C. B. Estudillo, and L. G.
Alpasan. 1995. Optimum stocking density
and tank size for larval rearing of the grouper Epinephelus coioides. A paper presented at the Fourth Asian
Fisheries. Forum 16-20 October 1995,
Beijing, PROC.
Duray, M. N., C. B. Estudillo, and L. G.
Alpasan. (in press, b). The effect of background color and rotifer
density on rotifer intake, growth and survival of the grouper (Epinephelus
suillus) larvae. Aquaculture.
Duray, M. N., C. B. Estudillo, and L. G.
Alpasan. (in press, c). Larval rearing of the grouper, Epinephelus
suillus under laboratory conditions.
Aquaculture.
Emata, A. C., B. Eullaran, and T. U.
Bagarinao. 1994. Induced spawning and early life description
of the mangrove red snapper, Lutjanus argentimaculatus. Aquaculture 121: 381-387.
Garcia, L. M. B. (in press). A review of SEAFDEC/AQD finfish breeding
research. In: C. L. Marte, G.
Quinitio and A. Emata
(eds.). Proceedings of the
Seminar-Workshop on Breeding and Seed Production of Cultured Fishes in the
Philippines. 4-5 May 1993, Tigbauan,
Iloilo, Philippines. SEAFDEC/AQD,
Tigbauan, Iloilo, Philippines.
Kunvankij, P., L. B. Tiro, B. P.
Pudadera, and I. O. Potestas. 1986. Induced
spawning and larval rearing of grouper (Epinephelus salmoides
Maxwell), p. 663-666. In: J. L. Maclean, L.B. Dizon and
L.V. Hosillos (eds.) The First Asian
Fisheries Forum. Asian Fisheries
Society, Manila, Philippines.
Marte, C. L., G. Quinitio, and N. Caberoy. 1995. Spontaneous
spawning of sex-inversed grouper Epinephelus coioides administered
17-alpha methyl-testosterone implants.
A paper presented at the Fourth Asian Fisheries Forum. 16-20 October 1995, Beijing, PROC.
Nagai, A. 1990. Natural spawning
and larval rearing of grouper, Epinephelus malabaricus. A terminal report submitted to SEAFDEC
Aquaculture Department, Tigbauan, Iloilo, Philippines. 40 pp.
Quinitio, G. F. and M. N. Duray. (in press).
Review of SEAFDEC/AQD Finfish Seed Production Research. In: C. L. Marte, G. Quinitio and
A. Emata (eds.). Proceedings of the Seminar-Workshop on
Breeding and Seed Production of Cultured Fishes in the Philippines. 4-5 May 1993, Tigbauan, Iloilo,
Philippines. SEAFDEC/AQD, Tigbauan, Iloilo,
Philippines.
Quinitio, G. F. and J. D. Toledo.
1991. Mariculture techniques for Epinephelus
sp. in the Philippines, P. 94-106.
In: R. D. Guerrero III and M.P. Garcia, Jr.
(eds.) Advances in Finfish and Shellfish Mariculture: Proceedings of the
First Philippine-French Technical Workshop on Advances in Finfish and Shellfish
Mariculture. 24-26 October 1990, Los Baños, Laguna, Philippines. Philippine Council for Aquatic and Marine
Research Development, French Embassy in the Philippines.
Quinitio, G. F., R. M. Coloso, N. B.
Caberoy, J. D. Toledo, and D. M. Reyes,
Jr. 1996, p. 103-107. In: MacKinlay D. and Eldridge M. (eds.). Egg quality of
grouper Epinephelus coioides fed different fatty acid sources. The Fish Egg: Its Biology and Culture Symposium
Proceedings. International Congress on
the Biology of Fishes. 14-18 1996, San
Francisco State University.
Shapiro, D. Y. 1987. Reproduction in
groupers. p. 295-327 In: J.
J. Polovina and S. Ralston (eds.) Tropical Snappers and
Groupers: Biology and Fisheries Management.
Westview Press, Inc., Boulder, Colorado.
Tan-Fermin, J. D. 1992.
Withdrawal of exogenous 17-alpha mehtyltestosterone causes reversal of
sex-inversed male grouper Epinephelus suillus (Valenciennes). The Philipp. Scientist 29: 33-39.
Tan-Fermin, J. D., L. M. B. Garcia, and
A. R. Castillo, Jr. 1994. Induction of sex inversion in juvenile
grouper. Epinephelus suillus,
(Valenciennes) by injections of 17Â-methyltestosterone. Japan. J.
Ichthyol. 40: 413-420
Toledo J. D., A. Nagai, and D. Javellana.
1993. Successive spawning of grouper, Epinephelus suillus
(Valenciennes), in a tank and a floating net cage. Aquaculture 115: 361-367.
Watanabe, T. 1985. Importance of the
study of broodstock nutrition for further development of aquaculture. p. 395-414.
In Cowey, C.B., Mackenzie, A.M., and Bell, S.G. (eds.). Nutrition and Feeding of Fish. Academic Press, London